Chemical
Chemical characteristics
Advantages
Disadvantages
Negatively charged
Good solubility – long half-life
Nuclease and RNase H resistant
Do not enter the heart
Toxicity
Uncharged
Relatively safe
Nuclease and RNase H resistant
Stable
No interaction with cellular proteins
No activation of innate immune responses
Does not enter the heart
Rapid renal clearance,
Poor cellular uptake
Cell-penetrating peptide-conjugated PMOs (PPMOs)
Conjugation of cell-penetrating peptides to PMOs
Enhanced cellular delivery
Effective in vivo delivery
Enter cardiac cells
Toxicity
Vivo-morpholinos (vPMOs)
Conjugation of dendrimeric octa-guanidine to PMOs
Enter cardiac muscle
Effective at lower dose
Toxicity
Tricycle-DNA (tcDNA)
tc-DNA deviates from natural DNA by three additional C-atoms between C(5′) and C(3′)
Increased RNA affinity and hydrophobicity Nuclease resistant Do not activate RNase H Enter the heart and cross blood–brain barrier [11]
Toxicity
1.
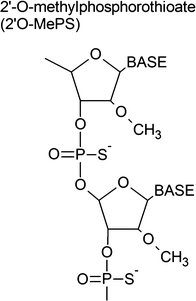
2′-O-Methyl-phosphorothioate oligonucleotide (2′OMePS)
Drisapersen (GSK2402968/PRO051) is a successful example of a 2′OMePS-based AONs targeting exon 51. Although statistically significant improvement in the 6-min walking distance test (6MWT; [12]) was not evident in the Phase III trial of drisapersen, BioMarin and Prosensa have recently announced completion of the submission of a New Drug Application (NDA) for drisapersen to the US Food and Drug Administration (FDA) (http://investors.bmrn.com/releasedetail.cfm?ReleaseID=908731). BioMarin also expressed its intention to submit a marketing authorization application for drisapersen in the European Union in the summer of 2015.
2.
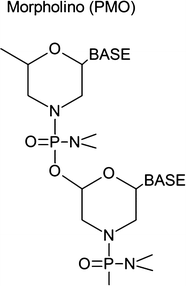
Phosphorodiamidate morpholino oligos (PMOs)
Eteplirsen (AVI-4658) (Sarepta Therapeutics, formerly AVI BioPharma, http://www.sarepta.com) is a morpholino-based AONs targeting exon 51 of the DMD gene. Among a variety of AONs tested for exon skipping in animal models, the PMOs is reported to be the safest. For example, mdx mice reportedly tolerate a 3-g/kg dose [13]. In human, Sarepta employed a high dose (30 or 50 mg/kg) of eteplirsen for a long period (48 weeks) to cause exon skipping in clinical trials (Phase IIb) on DMD patients without severe side effects [14].
Weaknesses of PMOs are that PMOs are poorly taken up by muscle fibers and are excreted in urine soon after administration. In addition, the uptake of PMOs by muscle is not uniform. It is suggested that the cellular uptake is a passive process, probably through leaky plasma membranes of the muscle fibers. Therefore, repeated administration of PMOs in high doses is required to sustain therapeutic levels of dystrophin expression. In addition, morpholino AONs do not enter cardiac cells.
Sarepta has completed Phase IIb studies and recently announced the results of long-term outcomes through 168 weeks of a Phase IIb study of eteplirsen. The results showed a statistically significant treatment benefit (http://phx.corporate-ir.net/mobile.view?c=64231&v=200&d=1&id=2006709). Sarepta now plans to submit an NDA by mid-2015 after collecting more data to meet FDA requirements.
3.
Modified PMOs
Unmodified PMOs require frequent (weekly or biweekly) high-dose injections to maintain therapeutic levels of dystrophin protein. In addition, PMOs cannot be delivered to cardiac muscle, which is an important therapeutic target for DMD. To avoid frequent administration and lower the dose for long-lasting therapy for DMD boys and hopefully target the heart, PMOs have been improved by organic chemists.
Cell-penetrating peptide-conjugated PMOs (PPMOs)
AVI-5038, targeting exon 50, is a PPMO developed by Sarepta. PPMOs are actively taken up by the cells. Therefore, compared to unmodified PMOs, far lower doses of PPMOs can restore sufficient dystrophin even in cardiomyocytes. A disadvantage of PPMOs is that they have dose-dependent toxicity. High doses of PPMO caused adverse effects, such as lethargy, weight loss, and tubular degeneration in kidneys of rats [15, 16].
Vivo-morpholinos (vPMOs)
vPMOs contain a cell-penetrating moiety, an octa-guanidine dendrimer. Due to this modification, vPMOs are actively taken up by cells. As expected, vPMOs are reported to lead to extensive and prolonged dystrophin expression in the dystrophic mdx mouse, an animal model of DMD, at a relatively low dose ([17–19]; Echigoya et al., [20]). vPMOs also effectively induced exon skipping in dystrophic dogs in vivo [21].
4.
Tricycle-DNA (tcDNA) oligomers
Goyenvalle et al. reported the development of tricycle-DNA (tcDNA) oligomers, as a promising tool for exon skipping therapy of DMD. Mdx mice treated with high doses of tcDNA AONs (200 mg/kg/week for 12 weeks) showed improved cardiac function and reduced freezing response [11], which characterizes the neurological defects of mdx mice [22]. The authors also reported that tcDNA has a higher propensity to spontaneously self-assemble into nanoparticles. The authors speculate that this property explains efficient uptake of tcDNA by the cells and therefore efficient exon skipping in vivo.
To achieve high concentrations of AONs in the muscle, further development of new types of AONs is in progress. Tissue-specific delivery systems for AONs are also important to achieve efficient and safe exon skipping with low doses of AONs.
13.3 Preclinical Studies Using Dystrophic Animal Models
13.3.1 Mdx 52 Mice
Although many exon skipping studies have been performed on mdx mice, which have a nonsense mutation in exon 23, a type of mutation that is rare in human, therefore, we used mdx52 mice, in which exon 52 has been deleted by a gene targeting technique [23] for preclinical study. We designed more than 10 AONs covering exon 51 of the mouse DMD gene and injected them intramuscularly into mdx52 mice. The highest splicing efficiency was generated by a two-oligonucleotide cocktail targeting both the 5′ and 3′ splice sites of exon 51. We systemically injected the cocktail into mdx52 mice seven times at weekly intervals. This protocol induced 20–30 % of wild-type dystrophin expression in all muscles and was accompanied by amelioration of the dystrophic pathology and improvement of skeletal muscle function [24]. This study provided proof of concept for exon 51 skipping in the DMD animal model. Then, we participated in one of the global trials of exon 51 skipping using drisapersen (Phase III). Exon 51 skipping is estimated to be applicable for up to 13 % of all DMD patients, representing the largest patient population treatable by a single-exon skipping strategy.
13.3.2 CXMDJ Dogs
We established a colony of dystrophic dogs (CXMDJ) in Japan [25]. The beagle is smaller and more widely used in pharmaceutical research than the golden retriever. Therefore, the original golden retriever muscular dystrophy dogs (GRMD) were crossed with beagles seven times. Using beagle-CXMDJ dystrophic dogs, which harbor a splice site mutation at the boundary of intron 6 and exon 7, we performed systemic administration of a mixture of AONs simultaneously targeting both exons 6 and 8 of the canine DMD gene. In treated CXMDJ dogs, truncated dystrophin proteins were expressed at the sarcolemma, and the performance of affected dogs was significantly improved without serious side effects [26]. The results convinced us that systemic delivery of PMOs for exon skipping is safe and effective, except to cardiac muscle. We also confirmed that the AONs that were effective in the dystrophic dogs also worked in cells from an exon 7-deleted DMD patient [27].
13.4 Phase I Investigator-Initiated Exploratory Study in NCNP Hospital
In 2009, we started development of AONs, which allow exon 53 skipping of the DMD gene together with Nippon Shinyaku Co., Ltd. (http://www.nippon-shinyaku.co.jp/english/). Targeting exon 53 is applicable to DMD patients with deletions of dystrophin exons 42–52, 45–52, 47–52, 48–52, 49–52, 50–52, and 52. In Japan, 300–400 patients are estimated to be treatable by exon 53 skipping. NS-065/NCNP-01 is a newly developed PMO-based AON that skips exon 53 of the dystrophin gene. After the efficacy and safety were confirmed in preclinical studies, a dose escalation study was designed to evaluate the systemic delivery of NS-065/NCNP-01 by intravenous infusion once per week for 12 weeks to three cohorts. The primary endpoint of the trial was safety, and the secondary endpoints were pharmacokinetics and dystrophin expression. Ten subjects, aged 6–14 years responsive to exon 53 skipping, were recruited through REMUDY (Chap. 11) and randomly allocated to one of the three cohorts. In vitro confirmation of exon 53 skipping and dystrophin recovery in subject-derived cells [27] was one of the inclusion criteria. At the end of 2014, administration to all subjects had been completed. The initial analysis detected dystrophin mRNA with the amino acid reading frame restored by exon 53 skipping in every dose group. Furthermore, the expression of dystrophin protein that appeared to have been translated from such an mRNA was detected in the high-dose group. In addition, no serious adverse event was observed throughout the study, and no subject discontinued administration. Anemia and increases in some renal parameters were observed as general adverse events. The analysis of data from the study is still ongoing and will be published elsewhere. As the next step, ambulatory patients should be treated with dose escalation. Using the results of the investigator-initiated clinical trial, Nippon Shinyaku is preparing for the next stage of clinical development with a view to launching this product in Japan in 2018.
13.4.1 Evaluation of the Efficacy of Exon Skipping
In our Phase I study, we assessed the safety of the drug as the primary endpoint. For approval of the drug, how to evaluate the clinical outcomes remains controversial. Although 6MWT, which measures the distance an individual is able to walk in 6 min on a hard, flat surface, is a well-accepted, widely used measurement of cardiac, respiratory, circulatory, and muscular function in DMD (https://www.thoracic.org/statements/resources/pfet/sixminute.pdf), whether 6MWT is suitable for primary endpoint to meet for future clinical trials for DMD is under discussion.
13.5 Next Strategy for Exon Skipping Therapy
13.5.1 Delivery to Cardiac Muscle
Many DMD patients die due to cardiac dysfunction. Since unmodified PMOs or 2′OMePSs do not enter cardiomyocytes, the development of new AONs that can reach cardiac muscle has been awaited. vPMOs or PPMOs are PMOs modified to be efficiently taken up by cardiac cells. In fact, these modified PMOs show higher efficiency in exon skipping in the heart than unmodified PMOs and 2′OMePSs. Importantly, however, modified PMOs have been reported to be cytotoxic at higher doses [16]. The mechanisms of the side effects of modified PMOs remain to be determined. In addition to AON chemistry, the development of a muscle-specific delivery system is important.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







