Toxoplasmosis
Ruth Lynfield
Folashade Ogunmodede
Nicholas G. Guerina
Toxoplasma gondii is an obligate intracellular protozoan parasite found in many animal species throughout the world. Nicolle and Manceaux observed the parasites in the blood, liver, and spleen of a North African desert rodent, Ctenodactylus gondii. In 1909, the parasite was named Toxoplasma (“arclike form”) gondii (after the rodent). In 1923, Janku reported cysts in the retina of an infant who had unilateral microphthalmia, hydrocephalus, and seizures. Subsequently, Toxoplasma was recognized as causing a variety of clinical syndromes in humans. Although infection usually is asymptomatic in normal hosts, serious disease may occur in the setting of congenital infection or in an immunodeficient host. Infection with Toxoplasma is lifelong. The acute stage of infection is characterized by parasitemia. The chronic stage occurs when the parasite becomes encysted in host tissues. Toxoplasma may break out of host cells, causing a local reactivation. In the setting of an inadequate immune response, reactivated disease may lead to systemic spread of the parasites.
LIFE CYCLE
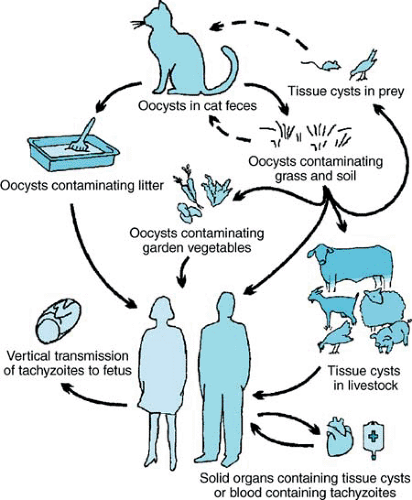
Toxoplasma has a sexual cycle that occurs exclusively in felines and an asexual cycle that occurs in most warm-blooded animals (Fig. 222.1). Cats acquire the infection by ingesting oocysts in contaminated soil or tissue cysts in birds and rodents they hunt and kill. The sexual cycle takes place in the cat intestine, where gametocytes are formed and fertilized to form zygotes. Zygotes become encapsulated within a wall and are shed as oocysts. During a primary infection, a cat can shed millions of oocysts daily for a period of 1 to 3 weeks. The oocysts sporulate and become infectious 24 hours or more after excretion. Sporulated oocysts may remain infectious in soil for more than 1 year, especially in warm, humid environments. Although cats typically develop immunity to T. gondii after a primary infection, the duration of immunity to intestinal shedding is not known; however, reinfection with oocyst shedding has been observed.
An animal or human may ingest material contaminated by cat feces containing Toxoplasma oocysts. The oocyst ruptures in the intestine and releases sporozoites. The sporozoites divide in the intestinal cells and associated lymph nodes. Tachyzoites, the rapidly dividing form characteristic of the acute stage of infection, are formed and are dispersed via blood and lymph. When an effective immune response is established, the parasites become localized in tissue cysts composed of components from both the parasites and infected host cells. Within the tissue cysts are slowly dividing forms of T. gondii called bradyzoites; tissue cysts are the form characteristic of the chronic stage of infection. Bradyzoites within intact tissue cysts can survive for the life of the host. Tissue cysts can be found in any organ, but most typically are found in the brain, eye, heart, and skeletal muscles. Occasionally, tissue cysts may rupture, releasing bradyzoites. In the presence of an intact immune response, infection usually is controlled. In immunodeficient hosts, reactivation of chronic Toxoplasma infection may occur, with bradyzoites transformed into tachyzoites, which may proliferate rapidly and induce tissue damage.
The infectious cycle may be perpetuated by tissue cysts when an infected animal is ingested by a carnivore; the bradyzoites are released, infect the intestinal epithelial cells, and are transformed into tachyzoites that disperse systemically. When an immune response is established, bradyzoites again are formed. Studies of cats indicate that the prepatent period (the time between infection and shedding of the oocysts) and the quantity of oocysts shed following a primary infection vary depending on the form of T. gondii ingested. Primary infection following ingestion of tissue cysts is associated with greater oocyst shedding and a shorter (3 to 10 days versus more than 18 days)
prepatent period compared with primary infection after ingestion of oocysts.
prepatent period compared with primary infection after ingestion of oocysts.
TRANSMISSION OF INFECTION
Toxoplasma oocysts, bradyzoites, and tachyzoites can all cause infection in humans. Typically, isolated cases are identified, but outbreaks of toxoplasmosis have been linked to the ingestion of contaminated water, soil, and undercooked hamburger.
Ingestion or inhalation of oocysts may occur after the handling of contaminated soil or cat litter or after ingestion of contaminated water or food (e.g., unwashed garden produce). Oocysts are very hardy and can resist drying or treating with disinfectants, alcohols (95% ethanol, 100% methanol), or 10% formalin. They may be inactivated by heating (66°C).
Transmission can occur by ingestion of tissue cysts present in undercooked meat (especially pork and mutton). The bradyzoites in tissue cysts can be destroyed by heating (66°C) or gamma irradiation. Generally, freezing meat at -20°C also will kill tissue cysts. In addition to ingestion, bradyzoites can be transmitted via transplant of an organ containing tissue cysts. Rarely, tachyzoites may be transmitted through a blood transfusion, by a laboratory accident, or by ingestion of unpasteurized (goat’s) milk. Tachyzoites are the most fragile infectious form and cannot withstand freezing and thawing, drying, or exposure to digestive enzymes. Fetuses may become infected through the placenta by tachyzoites after a primary maternal infection.
PATHOGENESIS
Clinical manifestations and the severity of disease after Toxoplasma infection are affected by the interplay between parasite and host. Strain virulence, size of inoculum, stage of parasite, and route of infection are important parasite variables. Host variables include competence of the immune response, integrity of mucosal and epithelial barriers, and age. In animal models, differences in susceptibility based on genetic background have been demonstrated. Phylogenetic and statistical analyses indicate that the population of T. gondii consists of three clonal lineages. The relationships between these lineages and human susceptibility to infection are being actively investigated.
EPIDEMIOLOGY
Although toxoplasmosis occurs throughout the world, the percentage of seropositive individuals differs greatly in different regions and generally increases with advancing age. High prevalence rates have been reported from parts of Europe, Central America, and Central Africa. Several different prevalence patterns are identified, depending on whether the source of transmission is primarily by oocyst, tissue cyst, or both. Infection from ingestion of oocysts occurs frequently in parts of the world with many outdoor-living cats and a warm, humid environment. In such areas as Central America, seropositivity begins at approximately 1 year of age, when children begin playing in oocyst-contaminated sand and soil, and it reaches 50% to 75% by adolescence. In other regions of the world, transmission occurs primarily through the ingestion of undercooked meat. In these areas, depending on eating customs, seropositivity may begin in adolescence (or sooner) and can continue throughout adulthood. In many parts of the world, the pattern is mixed. Studies in certain regions actually have found a decreasing seroprevalence. U.S. military recruits were found to have an overall seroprevalence of 9.5% in 1986 as compared with 14.4% in 1962, and studies of French pregnant women in Paris noted a drop from approximately 87% during the period from 1960 to 1970 to approximately 70% in 1985. Seroprevalence rates among women of childbearing age in Europe vary from 50% in Brussels, Belgium, to 11% in Norway. In the United States, based on information from the National Health and Nutritional Examination Survey (NHANES), seroprevalence rates have remained stable over the last 10 years. The most recent NHANES, conducted in 1999 to 2000, estimated the overall Toxoplasma immunoglobulin G (IgG) seroprevalence rate at 15.8% and the seroprevalence among girls and women 12 to 49 years old at 15%.
With few exceptions, congenital infection occurs in the setting of primary maternal infection during pregnancy. The risk of congenital infection depends on the risk of acute acquired infection during pregnancy. This risk, in turn, depends on the yearly seroconversion rate for the particular population and on the age of the pregnant woman. Fetal infection may occur after reactivation of disease in immunocompromised pregnant women, and there are rare reports in the literature of fetal infection following chronic toxoplasmosis in pregnant women with no known immune dysfunction. The incidence of congenital Toxoplasma infection in some European countries with very high seroprevalence rates has declined in recent years, possibly owing to aggressive screening recommendations and national prevention programs. Brazil has the highest reported incidence of congenital infection in the Americas; 1 per 3,000 live births. In Massachusetts, where screening for congenital Toxoplasma infection has been part of the newborn screening program since 1986, the estimated incidence is 1 per 10,000 live births.
CLINICAL MANIFESTATIONS
Acquired Infection in Immunocompetent Hosts (Including Pregnant Women)
Most cases of Toxoplasma infection are subclinical in individuals with normal immune systems, and usually disease is self-limiting. Lymphadenopathy is the manifestation recognized most frequently, and the location most often is cervical, followed by axillary and then inguinal sites, although any group of lymph nodes may be involved. Adenopathy may occur more commonly at single sites in adults, but is more likely to occur at multiple sites in children. Usually, the lymph nodes are firm and movable and initially may be tender. They do not suppurate, and typically the lymph nodes are 1 to 2 cm in size, but they may be as large as 6 cm. Most cases of lymphadenopathy resolve over the course of 1 to 2 months.
Occasionally, disease may persist beyond 6 months. Adenopathy may be recurrent. The differential diagnosis of toxoplasmic lymphadenopathy includes lymphoma, leukemia, and other malignancies, infectious mononucleosis (Epstein-Barr virus), cytomegalovirus (CMV) infection, human immunodeficiency virus (HIV) infection, cat-scratch disease, bacterial lymphadenitis, atypical mycobacterial infection, tuberculosis, tularemia, and sarcoidosis. Other clinical presentations include an infectious mononucleosis–like illness with fever, malaise, and myalgia, although sore throat and hepatosplenomegaly are not typical with Toxoplasma infection.
Ocular disease may be associated with acute acquired infection in normal hosts but is associated more typically with congenital disease and reactivated disease. Although it is rare, severe systemic disease, including encephalitis, has been reported in apparently normal children (see further discussion later in this section). Other reported manifestations have included maculopapular rash, hepatitis, pneumonitis, myositis, myocarditis, pericarditis, and meningitis. Infection in immunologically
normal hosts usually is self-limited. Severe disease more typically occurs in patients with a deficient immune system.
normal hosts usually is self-limited. Severe disease more typically occurs in patients with a deficient immune system.
Infection in Immunocompromised Hosts
Toxoplasmosis in immunocompromised hosts may result from acute or reactivated infection. Disseminated infection may occur with fever and involvement of any and all organs, including brain, heart, and lung. It may result from primary infection in patients who are receiving immunosuppressive therapy (e.g., organ or bone marrow transplant recipients), in patients who have malignant disease (especially reticuloendothelial) and are undergoing chemotherapy, or in patients with acquired or congenital immunodeficiencies. Primary infection can occur also in transplant recipients via an infected organ or bone marrow or through blood transfusion. Diagnosing disseminated toxoplasmosis in such patients may be difficult because signs and symptoms are not specific to toxoplasmosis. However, the condition should be considered as a diagnostic possibility because the infection is rapidly fulminant. Early treatment can improve the outcome. Reactivation of latent infection may occur in the setting of altered immunity, such as immunosuppressive therapy after a transplant or decreasing immune function in a patient with acquired immunodeficiency syndrome (AIDS). Clinical findings include any of the following: retinal disease, encephalitis, pneumonitis, myocarditis, or multiorgan disease.
Toxoplasma encephalitis (TE) is a common opportunistic infection in adult patients with AIDS (10% to 50% of those seropositive for Toxoplasma with CD4 counts of fewer than 100 cells/μL), and typically it results from reactivation of infection in the setting of poor cellular immunity. In contrast to its incidence in adults, TE is uncommon in children with AIDS, most likely because of the low incidence of T. gondii infection. TE may present with fever, headache, and focal neurologic signs. Disturbances of consciousness, confusion, motor impairment, seizures, impaired coordination, and focal weakness can occur. Usually, the course is subacute, but it may be fulminant and rapidly fatal. Generally, focal hypodense mass lesions with contrast enhancement are seen. Magnetic resonance imaging with enhancement is thought to be more sensitive in detecting lesions than is computed tomography. Often, lesions are found in the basal ganglia and corticomedullary junction of the cerebral hemispheres. Usually, multiple lesions are found, but a solitary lesion can occur. Marked edema often is present. In patients with disseminated toxoplasmosis in the brain, contrast enhancement may be absent. An image finding that is highly suggestive but only present in approximately 25% of cases is the asymmetric (eccentric) target sign. This lesion is characterized by a ring of contrast enhancement with a small eccentric nodule along the wall, giving it a target-like shape. In developing countries, where neuroimaging and other sophisticated techniques may not be readily available, HIV-positive individuals with suspected intracranial masses should be empirically treated for TE. The main differential diagnosis of TE is primary central nervous system (CNS) lymphoma, especially in the presence of a single lesion. Other possibilities include progressive multifocal leukoencephalopathy, bacterial abscess, cryptococcosis, cytomegalovirus infection, tuberculosis, and focal viral encephalitis. Patients with TE may have coexisting intracranial diseases. Another CNS manifestation of toxoplasmosis is myelopathy, which has been reported in patients with AIDS.
Although TE is rare in individuals with intact immune systems, a report reviewing TE in children found that 9 of 32 reported cases occurred in children who had no known immunodeficiencies. In eight of these immunocompetent children, TE occurred with primary infection (one unknown).
Pulmonary toxoplasmosis may be seen in patients with AIDS with decreasing CD4 cell counts (typically, fewer than 100 cells/μL) or other immunosuppressed patients (primarily reported in adults). The clinical picture of pulmonary toxoplasmosis is similar to that of pneumocystis, although usually the course is more rapid. Patients present with fever, cough, and dyspnea. Often, diffuse bilateral interstitial pneumonitis is seen on chest radiography (although variously a miliary pattern, multiple nodules, lobar infiltrates, and pleural effusions have been described). Often, patients have a high lactate dehydrogenase level. The organism may be found in a bronchoalveolar lavage aspirate and in biopsy specimen, or in both, using appropriate histologic staining techniques. Thorascopic or open lung biopsy may be required to make a diagnosis in some cases.
Congenital Infection
The risk of fetal infection with T. gondii increases, but the severity of disease decreases, with the gestational age at which acute maternal infection occurs. The onset of fetal infection by T. gondii also is delayed after an acute maternal infection. The rate of maternal-fetal transmission may be as low as 1% or less with maternal infection in the periconceptional period and can approach 90% or greater with maternal infection in late gestation. Average transmission rates are approximately 15% or less overall for the first trimester (rate rapidly increases after 10 weeks of gestation), 30% for the second trimester, and 60% for the third trimester and are a function of the increasing efficiency of the placenta. Studies of twins have shown that monozygotic twins have nearly identical infection rates but dizygotic twins do not, further underscoring the importance of the placenta in fetal infection.
Although rare, there have been case reports of congenital T. gondii infection when maternal infection was documented before conception. There has also been a case report of congenital infection that was thought to result from maternal reinfection during pregnancy. Congenital infection following chronic maternal infection is a more frequent occurrence in the setting of maternal immunosuppression, such as women receiving cytotoxic or corticosteroid therapy or women with HIV infection. In these women, symptoms of reactivated maternal toxoplasmosis may be absent, and the fetal transmission risk as a function of the degree of maternal immunosuppression is not known.
In the absence of treatment of the fetus with combination anti-Toxoplasma drugs, most fetuses infected early in pregnancy die in utero or in the neonatal period or have severe neurologic and ophthalmologic disease. Those infected in the second and third trimesters typically have mild or subclinical disease. The delay in maternal-fetal transmission appears to diminish with increasing gestational age. Although most fetal infections are likely to occur within several weeks, a delay of more than 3 months has been described.
Most newborn infants with congenital Toxoplasma infection have subclinical infection with no overt disease at birth, but indirect ophthalmoscopy may reveal ocular disease, and examination of the cerebrospinal fluid (CSF) and intracranial radiographic imaging may reveal abnormalities of the CNS. The New England Newborn Screening Program measures Toxoplasma-specific IgM on all newborn infants in Massachusetts and New Hampshire. Over a 6.5-year period beginning in 1986, 52 infants with congenital Toxoplasma infection were identified from 635,000 infants screened. Fifty of the 52 infants had normal routine newborn examinations, but after their T. gondii infection was identified through serologic screening, 48 had further testing that revealed abnormalities of either the CNS or the retina in 19 (40%). Most often, ocular lesions consisted of unilateral macular retinal scars, and CNS lesions were characterized by small, focal cerebral calcifications and mild to moderate elevations of CSF protein.
The principal clinical findings for infants and children with symptomatic congenital toxoplasmosis were described by Eichenwald in 1960. Such children were classified as having disease limited to the CNS and eyes (108 of 152) or more generalized (systemic) disease (44 of 152). The latter group evinced a lower incidence of CNS and ocular disease and a higher incidence of hepatosplenomegaly, lymphadenopathy, jaundice, and anemia. For all 152 infants, the incidences of neurologic and ocular abnormalities were as follows: intracranial calcifications, 37%; abnormal CSF profiles, 63%; chorioretinitis, 86%; convulsions, 41%; and hydrocephalus, 20%. Examples of brain and retinal lesions are shown in Figure 222.2.
Complications of Congenital Toxoplasma Infection in the Absence of Extended Treatment
Prospective studies have shown that although most infants with congenital Toxoplasma infection have mild or subclinical disease at birth, they remain at significant risk for long-term sequelae. Most studies have focused on the occurrence of ocular disease and show that the incidence of new-onset retinal lesions may approach 90% and that the risk for new lesions extends into adulthood. Severe visual impairment and blindness can occur. A few studies also have reported a significant incidence of neurologic problems, even when subclinical infection was present at birth. Specific complications include motor and cerebellar dysfunction, microcephaly, seizures, decreased intelligence quotient, mental retardation, and sensorineural hearing loss. Congenital toxoplasmosis has also been associated with precocious puberty secondary to hypothalamopituitary dysfunction.
Efficacy of Prophylaxis and Treatment of Congenital Toxoplasma Infection
Spiramycin has been shown to decrease in utero vertical transmission of Toxoplasma. In a prospective controlled study in France, the overall incidence of congenital infection was decreased from 58% to 23% when mothers were started promptly on spiramycin after seroconversion was identified. The severity of disease in those fetuses who became infected did not appear to be altered. The failure of spiramycin to prevent all cases of maternal-fetal transmission may result from the initiation of treatment after fetal infection has already occurred or from a demonstrated high variability of maternal serum and amniotic fluid spiramycin concentrations. In addition, some studies failed to demonstrate any difference in the rate of maternal-fetal transmission when groups receiving no antenatal treatment, antenatal spiramycin treatment, or antenatal pyrimethamine and sulfonamide-sulfadiazine were compared. Nonetheless, it is generally accepted that antenatal treatment is beneficial; initiating anti-Toxoplasma chemotherapy as soon as possible following maternal seroconversion (typically with spiramycin) and changing maternal therapy to a combination of pyrimethamine and sulfadiazine (plus folinic acid rescue) when infection is documented appear to decrease the risk for severe fetal disease significantly.
Experience with extended postnatal treatment regimens for congenital Toxoplasma infection is increasing. Although the optimal duration of therapy has not been determined, combination therapy for a 1-year period—most often with pyrimethamine (plus folinic acid) and sulfadiazine—appears to decrease the incidence of long-term complications significantly. The incidence of new-onset ocular disease may be decreased to as low as 10% after a 1-year treatment regimen, although longer-term follow-up (through adolescence) is needed for most studies. Significant neurologic complications are limited to children with compromising CNS disease at birth, although some of such children may do better than expected as compared with untreated children. The Chicago Collaborative Treatment Trial reported 37 congenitally infected infants who received extended treatment initiated within the first months of life. Thirty-four of these infants had signs of generalized or neurologic disease. Although some of these children had visual handicap, most did well in follow-up (mean period of follow-up, 3.5 years; longest, 10 years). Nineteen children (who did not have hydrocephalus) had normal or nearly normal neurologic function. Severe disabilities occurred in eight of ten children who had symptomatic hydrocephalus at birth and in two of eight who had symptomatic hydrocephalus identified in the first months of life. Risk factors associated with a poor outcome included delay in the diagnosis and initiation of treatment, prolonged uncorrected hydrocephalus, extensive visual impairment, and prolonged concomitant neonatal hypoxemia and hypoglycemia.
Ocular Disease
Toxoplasma is the most common cause of infectious chorioretinitis in immunocompetent children. In the past, ocular toxoplasmosis was thought to be primarily a sequela of congenital infection; however, more recent data suggest it may more frequently follow postnatally acquired infection. For example, Southern Brazil has a very high seroprevalence rate, and most Toxoplasma infections are thought to be acquired postnatally, yet 18% of the population has chorioretinal scars. Similarly, 20 of 97 (21%) individuals infected during a 1995 waterborne outbreak of toxoplasmosis in British Columbia went on to develop Toxoplasmic chorioretinitis. Strain or host differences or inoculum size may account for the high rate of acquired chorioretinitis in some settings. Usually, chorioretinal disease associated with acquired toxoplasmosis involves one eye. Disease associated with reactivation from congenital infection may be bilateral, although many cases are unilateral.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree