CHAPTER 18 Topical management
1. Identify three principles of wound management.
2. Describe the characteristics of a physiologic wound environment.
3. Identify at least five objectives in creating a physiologic wound environment.
4. Define the terms occlusive, semiocclusive, moisture retentive, primary dressing, and secondary dressing.
5. List two indications and one contraindication for each dressing category.
6. Describe the factors to consider when selecting a wound dressing.
Principles of wound management
Wounds do not occur as an isolated event within a patient. Consequently, the principles of effective wound management must incorporate a holistic approach that identifies and intervenes to minimize or abate the underlying etiology and any coexisting contributing factors. Box 18-1 lists the three principles of wound management and examples of how these principles can be addressed. To control or eliminate causative factors and potential cofactors, diagnostic or laboratory tests are often required. Failure to address causative factors will result in delayed healing or a nonhealing wound despite appropriate systemic and topical therapy. No dressing can compensate for an uncorrected pathologic condition. The various possible etiologies of acute or chronic wounds and the cofactors that impair wound healing are discussed throughout this textbook. This chapter focuses solely on the third principle of wound management: create a physiologic wound environment.
BOX 18-1 Wound Management Principles and Examples
TABLE 18-1 Objectives of a Physiologic Wound Environment
| Objectives | Interventions |
|---|---|
| Cover wound with dressings impermeable to bacteria to protect from outside contaminants, with the most appropriate dressing or combination of dressings based on wound assessment and overall goals for the patient Infection control precautions, no-touch dressing application Appropriate wound cleansing and debridement Antimicrobials when indicated Appropriate wound culture technique (see Chapter 16) | |
| Normal saline with 4–15 psi of pressure/force to remove debris without harming healthy tissue | |
| Most appropriate debridement method or combination of debridement methods based on patient’s condition and wound assessment (see Chapter 17) Method of debridement consistent with patient’s overall goals | |
| Dressing with high moisture vapor transmission rate will allow moisture to escape and evaporate to manage minimally exudative wounds Moderate to heavily exudative wounds require absorptive dressings Select topical dressings that maintain moist wound environment to prevent tissue desiccation | |
| Hydrating or absorbent-impregnated gauze for large, deep wounds Fluff packing material and loosely place into wound using cotton-tipped applicator Ensure packing material is in contact with wound edges and can be easily retrieved | |
| Appropriate dressing change frequency Cleansing with each dressing change Debridement and antimicrobials as indicated Charcoal dressings | |
| Semiocclusive dressings Nonadherent dressings Dressings that require fewer changes Pain control interventions as described in Chapters 25 and 26 | |
| Skin barriers (liquid, ointments, wafers) to protect the periwound skin from moisture and adhesives as described in Table 5-4 Appropriate interval for dressing changes so that exudate does not pool on surrounding skin or undermine adhesive of wound dressing |
Characteristics of a physiologic wound environment
Topical wound management is the manipulation of the wound to restore a physiologic wound environment; an environment that is “characteristic of an organism’s healthy or normal functioning.” Key features of a physiologic wound environment are adequate moisture level, temperature control, pH regulation, and control of bacterial burden. These various conditions create a milieu that is conducive to a successful and expedient journey from compromised skin to skin repair and restoration of function. Wound care dressings are used to mimic the skin so that a physiologic local wound environment can be created. Objectives and interventions for creating a physiologic wound environment are given in Table 18-1.
Moisture level.
The human body is more than 65% water; the primary means of maintaining this level of moisture is located within the epidermis (Spruitt, 1972). The stratum corneum layer of the epidermis prevents loss of excessive amounts of water in the form of water vapor to the external environment. Therefore, moisture levels of healthy skin are maintained by an intact stratum corneum. When the stratum corneum has been removed or compromised, tissues and cells are subject to increased loss of moisture and may desiccate and eventually die. Wound healing is slowed significantly in such a dry environment because epithelial cells must burrow below the dry surface to reach a moist surface over which they can migrate (Hinman and Maibach, 1963; Winter, 1962). A moist environment physiologically favors cellular migration and extracellular matrix formation which facilitates healing of wounds, reduces pain and tenderness, reduces fibrosis, decreases wound infection and produces a better cosmetic outcome (Hopf et al, 2006; Robson et al, 2006; Schultz et al, 2003; Steed et al, 2006; Whitney et al, 2006). However, the moisture level of the wound surface can range from dry (particularly nonviable tissue) to an excessively moist surface. Topical dressings are used to establish and maintain a moist—not wet—wound environment. Topical wound dressings are used to maintain adequate moisture in the wound by one of three mechanisms: containing wound fluids, absorbing excess moisture, or donating moisture. Semiocclusive dressings can keep a wound moist, even when no additional moisture is supplied, by “catching” and retaining moisture vapor that is being lost by the wound on a continual basis. Their ability to maintain tissue hydration can be characterized by a measurement known as the moisture vapor transmission rate (MVTR). Dressings transmit less moisture vapor than the average wound loses, thus facilitating moisture retention in the tissue as opposed to desiccation. In general, if the dressing material transmits less moisture vapor than the wound loses, the wound will remain moist. If the dressing material transmits more moisture vapor than the wound loses, the wound may dry out. For this reason, transparent dressings designed for intravenous sites have a higher MVTR than the transparent dressings intended for wound management.
Normal temperature.
Another characteristic of a physiologic wound environment is a normal body temperature. One of the functions of the skin is to provide thermoregulation. The wound dressing must restore the insulating effect formerly provided by the skin. All cellular functions are affected by temperature, including chemical reactions (e.g., metabolism, enzymatic catalysis, production of growth factors interleukin-1β and interleukin-2, protein synthesis, and oxidation) and processes (e.g., phagocytosis, mitosis, and locomotion). Local hypothermia is known to increase the risk of infection by causing vasoconstriction and increasing hemoglobin’s affinity for oxygen, both of which result in a decreased availability of oxygen to phagocytes. The consequences of hypothermia on phagocytes include decreased phagocytic activity, decreased production of reactive oxygen products, and impaired ability to migrate. Topical wound dressings that reduce moisture loss from wounded tissues and do not require frequent changes will diminish local cooling (Wenisch et al, 1996).
Bacterial balance.
The importance of bacterial balance for a physiologic wound environment and interventions to control bioburden are discussed in Chapters 16 and 17. Strategies include (1) debridement, (2) appropriate wound cleansing, (3) appropriate infection control precautions, 4) use of antimicrobials, and (5) use of moisture-retentive dressings.
Semiocclusive dressings reduce wound infections by more than 50% compared with traditional gauze dressings. This finding supports the theory that semiocclusive dressings optimize the phagocytic efficiency of endogenous leukocytes by maintaining a moist wound environment and reduce airborne dispersal of bacteria during dressing changes. Semiocclusive dressings provide a mechanical barrier to the entry of exogenous bacteria. In contrast, bacteria have been reported to penetrate up to 64 layers of gauze (Ovington, 2001).
pH.
A neutral pH similar to the pH of blood (7.4), is required to provide a physiologic wound environment. In a wound, the pH of the tissue becomes mildly alkaline due to the loss of moisture. The presence of urine, stool, or fistula drainage in a wound will affect the local pH as well. The consequence of an altered pH is an increased the risk of bacterial invasion and impaired function of matrix metalloproteinases (MMPs) (Armstrong and Jude, 2002; Ovington, 2002). Semiocclusive wound dressings facilitate a mildly acidic to neutral wound fluid pH (Varghese et al, 1998).
Types of dressings
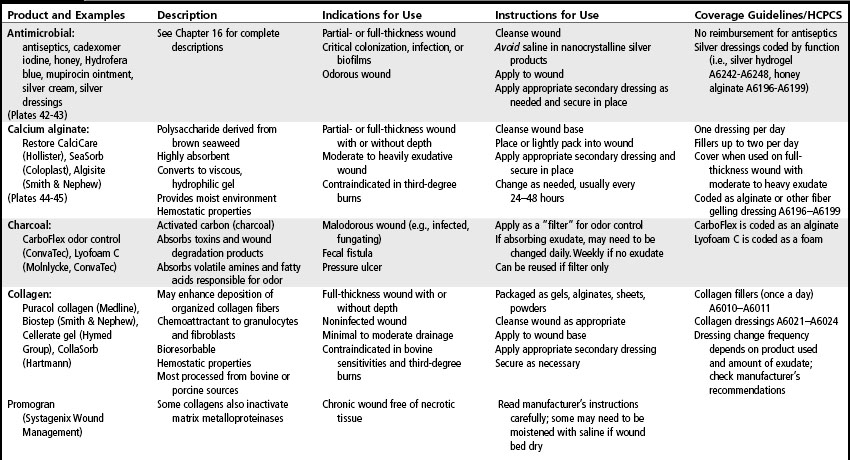
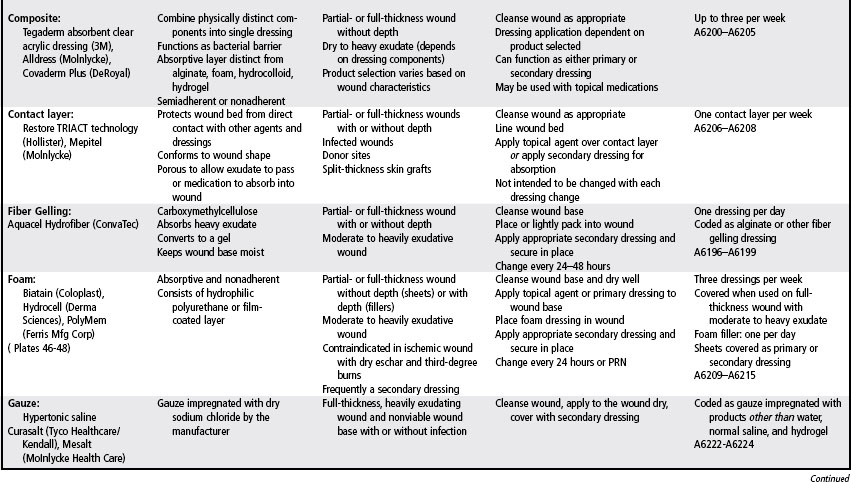
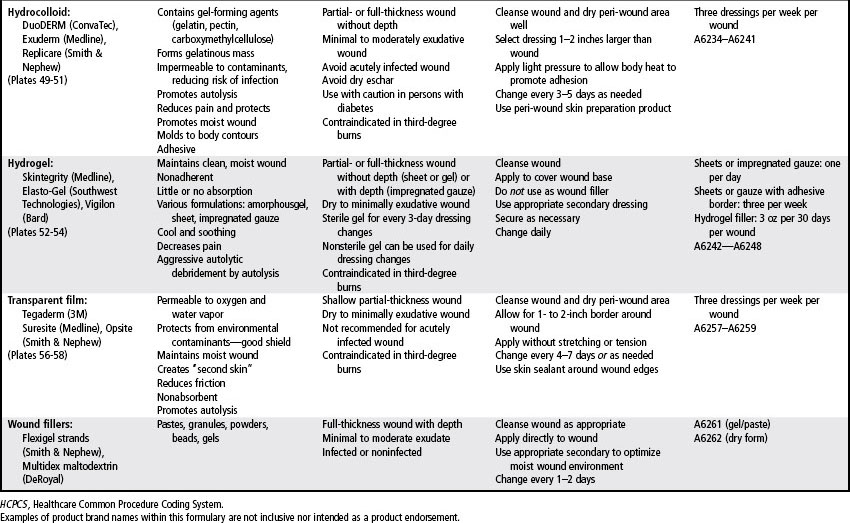
Most wound dressings today are semiocclusive rather than occlusive. Semiocclusive dressings (also known as moisture-retentive dressings) emerged in the 1970s and currently are staples in the wound care portfolio. Wound care technologies were worth more that $2 to $3 billion in 2005 and are predicted to reach $4.6 billion in 2011 (Andrews, 2001). The wound care market is constantly changing with an extensive variety of dressings that vary by shape, size, and ingredients. They are selected based on the needs of the wound, the patient, and the care setting.
Common ingredients in wound dressings include glycerin, polymers, carboxymethylcellulose, collagen, alginate, cellulose, cotton or rayon, and polyurethane. Wound care dressings may be single-component dressings containing, for example, only alginate, hydrogel, or hydrocolloid, or multicomponent dressings in which components are mixed together, such as an alginate and a hydrocolloid to increase absorptive capacity. Multicomponent dressings are categorized and reimbursed according to the clinically predominant component (e.g., alginate, collagen, foam, gauze, hydrocolloid, hydrogel). Most of the dressing categories are available in an antimicrobial form such as silver (see Chapter 16), however reimbursement is still based upon their clinically prominent component). The effects of these products and components have been reported by a number of studies (EPUAP-NPUAP, 2009; Hopf et al, 2006; Robson et al, 2006; Steed et al, 2006; Whitney et al, 2006).
Wound care needs may change frequently throughout the various phases of wound healing. Therefore, a variety of products must be accessible. In general, dressing selection and utilization is guided by the Medicare Part B Surgical Dressing Policy. Table 18-2 is a formulary of wound care products and provides examples in addition to the Medicare Part B utilization parameters and codes. Before using specific products, providers should refer to the manufacturer’s product insert for the most current information on contraindications, interactions, and utilization. The policy also contains a category for pouching wounds. These products are described in Chapter 38.
Alginate and fiber gelling
These highly absorptive products are gelling dressings made of spun fibers of brown seaweed (alginate dressings) or carboxymethylcellulose (fiber gelling dressings). They act via an ion exchange mechanism to absorb serous fluid or exudate, forming a nonadhesive, nonocclusive hydrophilic gel that conforms to the shape of the wound. These versatile dressings are available in the form of sheets or ropes that can be cut to the size of the wound or loosely packed into wound dead space (see Plates 44–45. Because these dressings absorb and hold exudate, they create a moist environment, thus promoting autolysis, granulation, and epithelization. Alginates have hemostatic properties as well.
Alginates and fiber gelling dressings are primary dressings changed as often as daily, or they are left on for several days depending on the volume of exudate and the secondary dressing used. For example, transparent films used as secondary dressings will not add to the absorptive capacity of an alginate or fiber gelling product, whereas foams used as secondary dressings with alginates will increase overall absorptive capacity and facilitate a longer wear time. Some alginates become almost amorphous gels in the wound and will require irrigation for removal, whereas others retain their structural integrity as they “gel” and can be lifted out of the wound. Removal of the dressing need not be aggressive, as the residual fibers are biocompatible. If an alginate is used inappropriately (e.g., used with minimally exudative wounds), the wound bed may become desiccated with alginate fibers imbedded in the wound. The alginate dressing should not be moistened prior to application. If the dressing is dry at the scheduled dressing change, the dressing change frequency should be decreased or an alternative product selected (Belmin et al, 2002; NPUAP-EPUAP, 2009).
Collagen
Collagen is a body protein that is degraded in chronic wounds by proteases and elastase. Collagen dressings usually are formulated with type I bovine (cowhides or tendon) or avian collagen or with type III porcine collagen. Collagen products are nonadhesive, nonocclusive primary dressings available in a variety of formulations: particles encapsulated in nonadherent pouches or vials, gels loaded in a syringe, pads, and freeze-dried sheets. Collagen dressings chemically bind to members of the MMP family, thus reducing the levels of proteases in chronic wounds (Cullen, 2002; Nisi et al, 2005; Ovington, 2002). A collagen dressing requires the use of a secondary cover dressing and can be safely used for a partial-thickness or full-thickness wound with granular or nonviable tissue. International pressure ulcer guidelines recommend considering the use of collagen dressings for nonhealing Stage III and IV pressure ulcers (NPUAP-EPUAP, 2009). However, they also describe a randomized controlled trial comparing collagen and hydrocolloids dressings that found no significant difference in the healing rate of Stage II and III pressure ulcers after adjusting for baseline depth (Graumlich et al, 2003). The guidelines also point out that collagen dressing are more expensive and time consuming to apply than hydrocolloid dressings.
Contact layers
Soft silicones are polymers, are insoluble to wound exudate, are not intrinsically absorbent, and do not interact chemically with the wound. Soft silicone dressings are manufactured as single contact layers, or they are added the wound-facing layer of an absorbent dressing (e.g., foam) for management of an exuding wound (Dykes and Heggie, 2003). An international advisory group of scar management experts recommends the use of silicone gel sheeting for the prevention and treatment of hypertrophic scars and keloids in select situations (Mustoe et al, 2002). Silicone dressings are easily removed and therefore do not traumatize the wound or the surrounding skin (NPUAP-EPUAP, 2009; Mustoe, et al 2002).
Foam
Polyurethane foam dressings are sheets of foamed polymers that contain variably sized small open cells capable of holding wound exudate away from the wound bed. A moist environment is created by maintaining the exudate in the wound space. Foam dressings are adhesive or nonadhesive, primary or secondary dressings, available in widely variable formulations (see Plates 46–48). Nonadhesive foam dressings can be secured in position with a secondary dressing, such as a transparent film dressing, or a nonadhesive wrap, such as roll gauze. The foam may be of traditional thickness (4–7 mm) or thin (<1 mm). Generally, the thin foam dressing has an adhesive wound surface and an outer layer of a transparent film dressing that provides a waterproof barrier. The traditional-thickness nonadhesive foam dressings may or may not have an adhesive border or an outer transparent layer that helps prevent strikethrough exudate. Foams are available as wafers, rolls, and cavity dressings as well as shapes to fit the heel, elbow, or sacrum. Polymeric membrane foams combine glycerin (to soften devitalized tissue), starch (to wick away exudate), and a surfactant (to loosen necrotic tissue) (Yastrub, 2004).
Because traditional-thickness open-cell foam is one of the most absorbent materials available, foam dressings are appropriate for moderate to heavy exudative wounds with or without a clean granular wound bed (Diehm and Lawall, 2005). International pressure ulcer guidelines recommend considering foam dressings for use on exudative Stage II and shallow Stage III pressure ulcers (NPUAP-EPUAP, 2009). The traditional-thickness nonadhesive foam dressing is particularly useful for management of exudate under compression in venous ulcers and in any wound with friable or fragile periwound skin. Foam dressings can be used as secondary dressings to absorptive primary dressings (e.g., alginate, collagen, or fiber gelling) to enhance absorption of wound exudate. When applied to a dry or minimally exudative wound, the traditional-thickness foam dressing may adhere to the wound surface and require irrigation to facilitate removal. Therefore only very thin foam can be used to manage the superficial wound with minimal exudate and protect intact vulnerable skin.
When a cavity foam dressing is used to fill depth, the size of the product should be determined carefully, leaving sufficient room in the wound for the cavity dressing to expand as it absorbs wound exudate. Single small pieces of foam dressings should not be placed in cavities (NPUAP-EPUAP, 2009). Foam dressings do not redistribute pressure but have been shown to be effective in reducing shear (NPUAP-EPUAP, 2009; Ohura et al, 2005). Foam dressings are not indicated for wounds with tunneling.
Hydrocolloid
Hydrocolloid dressings are a formulation of elastomeric, adhesive, and gelling agents. The most common absorbent ingredient in the hydrocolloid is carboxymethylcellulose, which was adapted from ostomy skin barriers. Multiple meta-analyses confirm what is widely accepted in clinical practice, that hydrocolloids result in better (statistically significant) wound healing outcomes compared to gauze (Bouza et al, 2005; Bradley et al, 1999; Singh et al, 2004). Studies have also shown that hydrocolloids result in healing rates similar to those of comparable such as alginates and foams or more advanced dressings such as collagens. (Bale et al, 1997; Belmin et al, 2002; Graumlich et al, 2003).
As a primary dressing, the hydrocolloid is available as a wafer in several shapes and sizes. Contoured dressings also are available that enhance adherence to anatomically challenging sites, such as the sacrum, heel, knee, and elbow (see Plates 49–51). Many hydrocolloid dressings have an adhesive border that extends beyond the actual hydrocolloid surface. This design prevents shear and friction from loosening the edges of the hydrocolloid and circumvents the need for additional tape along the borders of the dressing.
Traditional-thickness hydrocolloid dressings are indicated as primary dressings for minimally to moderately exudate partial- and full-thickness wounds without depth. Hydrocolloids can be used as a secondary dressing over filler materials such as hydrocolloid powders, pastes, and alginates. International pressure ulcer guidelines advocate the use of hydrocolloids for clean Stage II and noninfected shallow Stage III pressure ulcers in anatomic locations where the product does not roll or melt (NPUAP-EPUAP, 2009). Due to its prescribed wear time and reimbursement, wounds that require assessment more often than twice per week should not be treated with a hydrocolloid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree