Chapter 44 The Management of the Failed Total Elbow Arthroplasty
Background
Understanding the mechanism of failure of an elbow arthroplasty is critical to planning and treatment of the patient. The first distinction to make is between septic and aseptic failure. With septic failure, eradication or effective chronic suppression of the infection is necessary. Eradication is achieved with antibiotics, surgical debridement and often staged implant revision or excisional arthroplasty. Among aseptic causes, loosening from osteolysis due to polyethylene debris is the most common cause of long-term failure.1 Instability, periprosthetic fracture and material failure including implant fracture are other important failure modes requiring revision arthroplasty.
Presentation, investigation and treatment options
Presentation
Infection
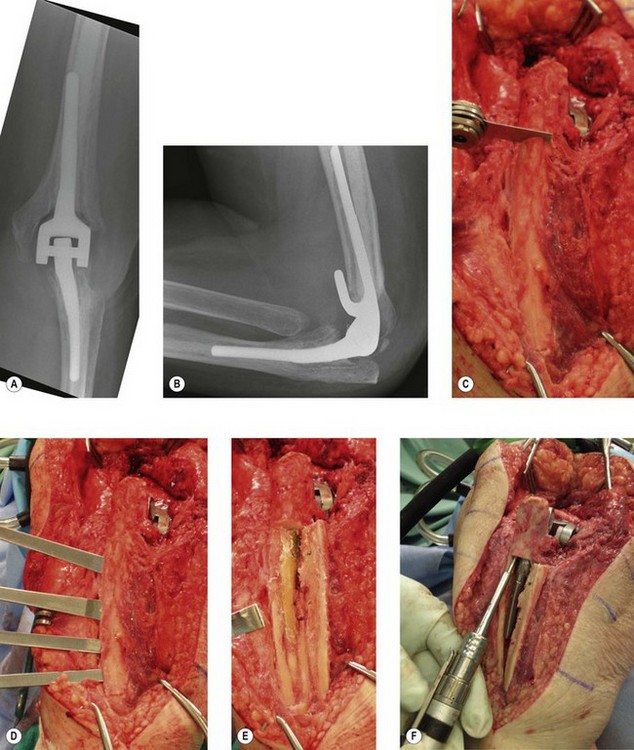
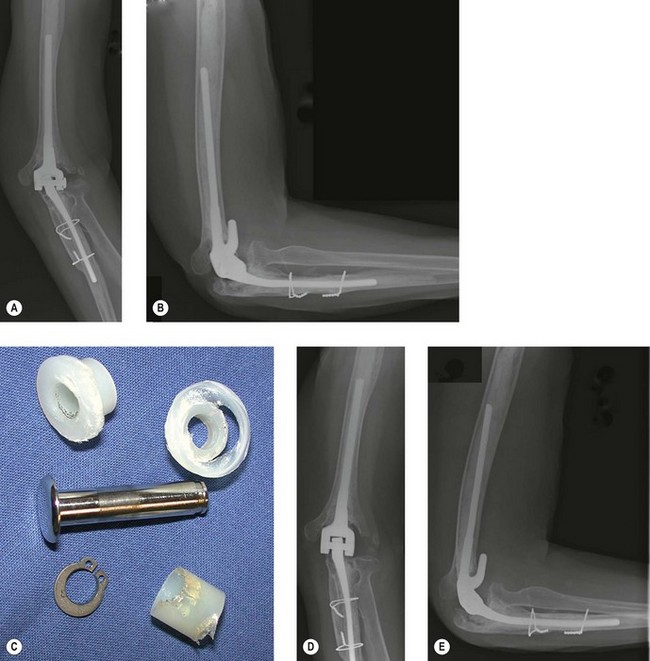
It is important to exclude an occult infection when evaluating a failed elbow arthroplasty, as it is difficult to eradicate and requires a different treatment plan than other modes of failure (Fig. 44.1). Infection rates following primary elbow arthroplasty vary from 1% to 12.5% in the literature with more recent series reporting a lower incidence than in the past.1–5 Risk factors for infection include a history of a prior infection, previous elbow surgery, severe rheumatoid arthritis, prolonged postoperative wound drainage, spontaneous drainage after postoperative day ten, elbow reoperation for any reason and psychiatric illness.2 Other factors associated with infection include immunosuppression secondary to diabetes, illness, various medications and psoriatic arthritis. Individuals with multiple risk factors such as a rheumatoid patient with previous elbow surgery have an even higher risk of infection reported to be as high as 31% in one series.2
The three factors to consider when managing an infected elbow arthroplasty are the organism involved, fixation of the implant and the time from symptom onset to treatment. The most common infecting organisms are Staphylococcus aureus and Staphylococcus epidermidis,1,2,5–7 however, other organisms have also been reported including Klebsiella, Enterococcus, Pseudomonas and haemolytic Streptococcus. Staphylococcus epidermidis infections have been associated with a high incidence of recurrent infection following revision, often requiring excisional arthroplasty.1 This may be due to the ability of Staphylococcus epidermidis to produce biofilms, preventing eradication of the bacteria by appropriate antibiotics.
The state of implant fixation to bone is also important. Implant salvage with aggressive irrigation and debridement with polyethylene exchange is more likely to be successful in eradicating infection if the implant is well fixed than if the implant is loose.1 If an implant is well fixed, an initial attempt to retain the components with irrigation and debridement can be considered. If the prosthesis is loose then the recommended management is a two-stage revision with debridement and removal of implants followed by a subsequent reimplantation.
The third important factor is the time between onset of symptoms and surgical treatment. In the past, infection has been classified from the time of index arthroplasty to onset of infective symptoms: acute (less than 3 months); subacute (between 3 months and 1 year); or late (over 1 year). However, this staging has not been shown to correlate with outcome.1 Infection is better defined by the interval between onset of symptoms and treatment, as either acute (less than 30 days) or chronic (more than 30 days). With the exception of Staphylococcus epidermidis, prosthesis retention with irrigation, debridement and polyethylene exchange in the presence of a well-fixed implant, is more likely to be successful with acute as opposed to chronic infection.1,2
Aseptic loosening
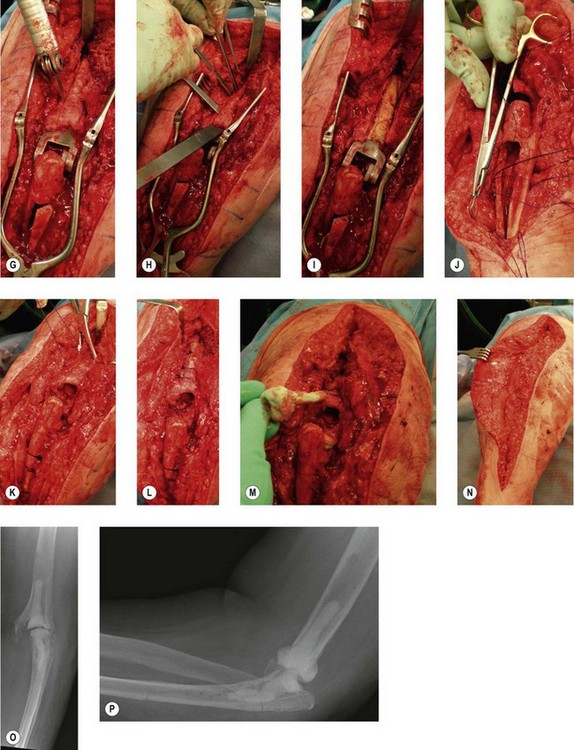
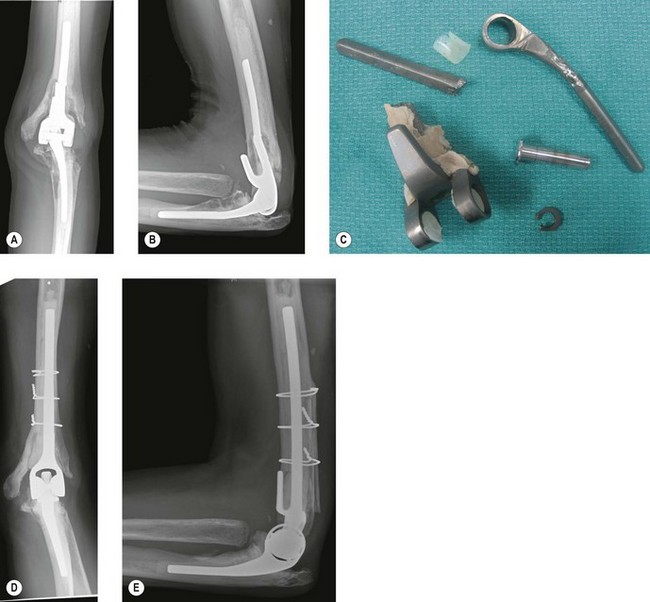
Aseptic loosening is the most frequent mode of failure for total elbow replacements8,9 (Fig. 44.2). The incidence depends on the indication for surgery, the type of implant used and the level of patient activity.10 Presentation is usually an insidious onset of activity-related pain in a patient with a previously well-functioning prosthesis. The pain gradually becomes more constant and eventually becomes functionally limiting. There may also be mechanical features such as audible or palpable crepitations. With severe osteolysis, periprosthetic fractures can occur with minimal trauma presenting acutely as a sudden increase in pain and deformity.10 Therefore, patients with asymptomatic osteolysis and implant loosening seen on radiographs should be seen regularly with interval radiographs to monitor the rate of periprosthetic bone loss.
The incidence of aseptic loosening is higher with more constrained prostheses.10,11 Patients who have had an elbow replacement for posttraumatic arthritis and those who continue to be very active with the elbow also have a higher rate of loosening.3,4,10 The Mayo Clinic has reported a revision rate of 19% at a mean follow-up of 6.3 years for posttraumatic arthritis compared to 2.6% at 10 years in rheumatoid arthritis.4 Similarly, other authors have reported a survival rate of 73% at 3 years and 54% at 5 years for posttraumatic arthritis, fractures and non-unions compared with 92% and 90% for inflammatory arthritis at those same time intervals.5 A recent study reported a 40% failure rate for posttraumatic arthritis and 19% for rheumatoid arthritis at an average 7-year follow-up.12
A retrieval study of failed primary total elbow semiconstrained components from aseptic loosening has identified four modes of wear that helps us better understand the modes of failure in elbow arthroplasty:13
Instability
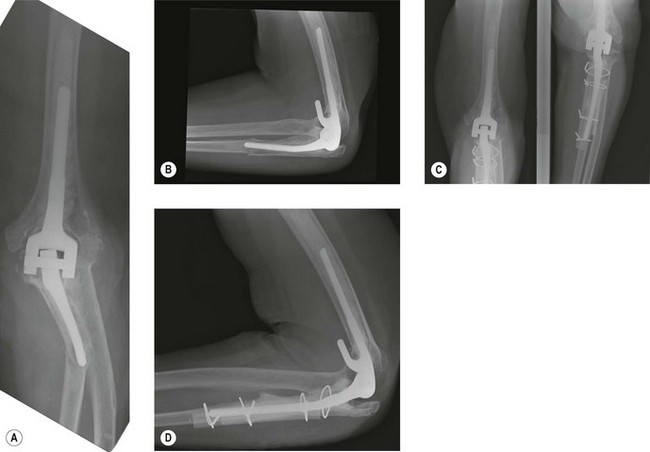
Failure due to instability is usually seen with unlinked total elbow designs with an incidence ranging from 0% to 13%14 (Fig. 44.3). Instability may also be seen in linked designs if the polyethylene linkage mechanism is worn. Instability may be classified as immediate (perioperative), early (less than 6 weeks postoperative) or late (more than 6 weeks postoperative).14 Acute and early instability is usually caused by component malpositioning or failed collateral ligament or triceps repairs and typically presents with a dislocation that can be easily diagnosed clinically and radiologically. Late instability may present with a dislocation but more commonly manifests as insidious symptoms of weakness, pain, giving way, clunking or other mechanical symptoms. Late instability may be secondary to component wear, malalignment or following a traumatic ligament injury.14
Implant material failure
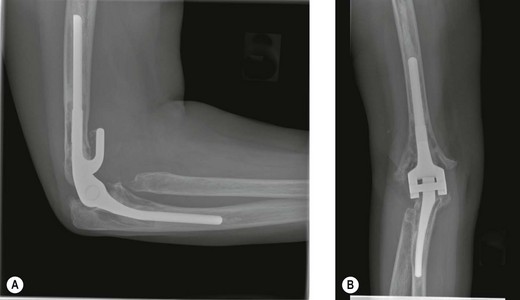
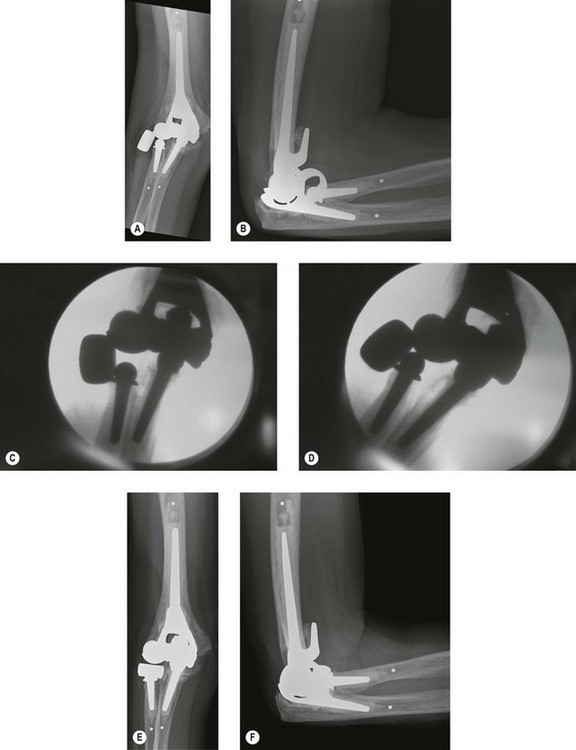
Implant material failure encompasses progressive polyethylene bearing wear, linkage mechanism failure and implant fracture (Fig. 44.4). Most commonly there is progressive mode 1 and 3 wear of the polyethylene bearing which in turn leads to polyethylene fracture and mode 2 and 4 wear. Wear debris contributes to progressive osteolysis and aseptic loosening of the implant. This may present as progressive joint instability with discomfort, squeaking or dislocation of the prosthesis if the arthroplasty depends on the polyethylene to stabilize the elbow.6,15 Factors thought to play a role in increased bearing wear include a high activity level, significant preoperative deformity and malalignment of linked prostheses, all of which result in excessive forces and moments being transmitted to the articulation.2,14 Other factors include young age, males, posttraumatic arthritis and supracondylar non-union.16,17
Stem fracture is related to implant design and malalignment, excessive patient activity and traumatic events.3,4,10,14,18 Stem fracture is uncommon with an incidence of 1.2% for the ulnar component and 0.65% for the humeral component of the Coonrad–Morrey prosthesis18 (Fig. 44.5). Osteolysis predisposes the stem to fatigue fracture as a result of compromised support of the stem. The component will break through the weakest part of the unsupported stem.19 The bearing mechanism may also fail acutely with backing out or breakage of the axis pin or C-ring.8,16 Linkage failure presents as a sudden onset of pain, deformity, joint instability or dislocation and limited range of motion.
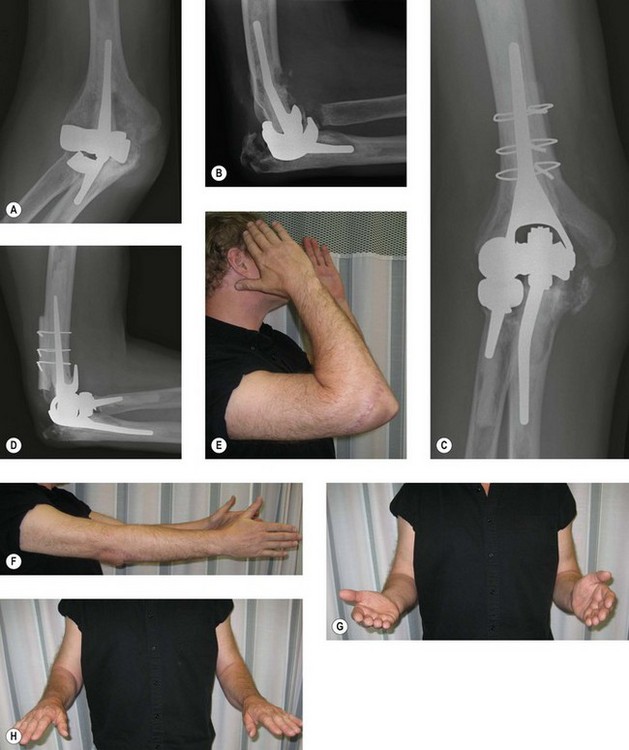
Periprosthetic fracture
Periprosthetic fracture can occur intraoperatively during implantation, or postoperatively either from a single traumatic event or secondary to significant osteolysis10 (Fig. 44.6). The reported rates of postoperative periprosthetic fracture vary between 6% and 22%.3 The three factors to consider in the management of periprosthetic fractures are the fracture location, implant fixation and the quality of the remaining bone stock. Management strategies for periprosthetic fractures will be discussed later in the chapter.
Investigation
History
Imaging
Anteroposterior and lateral radiographs of the humerus, elbow and forearm should be undertaken. Oblique views can be used if needed to further evaluate for any cortical perforations and cement extrusion. It is also important to have previous radiographs for comparison. Fluoroscopy can be used to evaluate joint stability and implant fixation14 (Fig. 44.7). CT scans are seldom necessary but can be useful to further delineate bone loss in spite of significant metallic artefact.
The plain radiographs should be carefully studied for implant and bony alignment, osteolysis, cortical perforations, periprosthetic and prosthetic fractures, bone loss, cement extrusion and the presence of a proximal humeral prosthesis. All these factors will affect the management of a failed prosthesis. Aseptic loosening appears as a gradually progressive radiolucent zone around the implant as seen on plain radiographs. These lines represent osteolysis at the bone–cement or cement–implant interface or bone–implant interface if the prosthesis is uncemented. Osteolysis can be graded at the bone–cement interface according to the system developed by Morrey et al:10
Gross loosening is usually associated with severe osteolysis and definite loosening can be diagnosed if there are cracks in the cement mantle or if the position of the implant has shifted within the bone. An alternative method of describing lucencies around implants was described by Trail et al using a zonal system on anteroposterior and lateral radiographs with five zones surrounding the distal humerus and five zones surrounding the proximal ulna.20
Bone loss is common and an important technical concern in revision total elbow arthroplasty. The type of implant and supplementary bone graft to manage this problem will depend on the location and extent of this loss. A grading system for bone loss has been developed by Mansat and coworkers.21 For the humerus:
For periprosthetic fractures, the system of radiographic grading was described by Sanchez-Sotelo et al22 (Fig. 44.8). For the humerus:
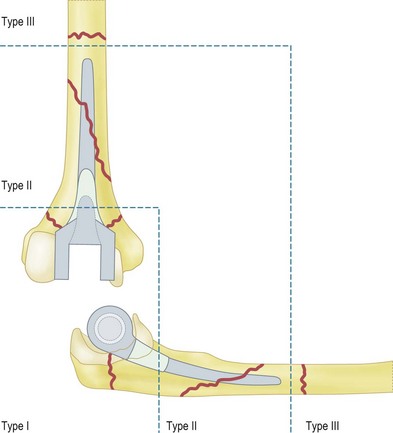
Figure 44.8 Classification of periprosthetic fractures. See text for details.
(Redrawn with permission of Sanchez-Sotelo J, O’Driscoll S, Morrey BF. Periprosthetic humeral fractures after total elbow arthroplasty: Treatment with implant revision and strut allograft augmentation. J Bone Joint Surg (Am) 2002; 84:1642–1650.)
Additional investigations for infection
A white cell count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) should be routinely performed for all failed or failing elbow arthroplasties. The white cell count may be elevated in an acute infection but is typically not in the setting of chronic infection.2 ESR and CRP are markers of inflammation and can be elevated in infection, with inflammatory arthritis or, indeed, in a normal postoperative course. In lower extremity arthroplasty, these values peak at postoperative days 2–3. The CRP will continue to be elevated for up to 3 weeks and the ESR for several months.23 However, persistent elevation of these markers outside of this timeframe is suggestive of either infection or inflammatory arthritis. A joint aspiration should be performed when the surgeon is suspicious of infection. The aspirate should be studied for Gram staining, culture and sensitivity and a white cell count. A 10-day extended culture is recommended to identify Propionbacterium acnes, which is increasingly being recognized as a cause of upper extremity joint infections. A white cell count of over 25 000/mm3 or a differential of over 75% polymorphonuclear leucocytes (PMNLs) is highly suggestive of infection.24 Ideally this aspiration should not be performed within 4 weeks of antibiotic treatment to lessen the chance of a false-negative culture. Intraoperatively, tissue should also be sent for Gram staining, culture and sensitivity as well as frozen section. Greater than 10 PMNLs per high power field seen on frozen section is highly suggestive of infection.
Summary Box 44.1
Treatment options
Infection
The primary objective of treating an infected total elbow replacement is to eradicate the infection (Fig. 44.1). The treatment options are long-term antibiotic suppression, implant retention with debridements, single-stage revision, excisional arthroplasty alone or excisional arthroplasty with delayed reimplantation (two-stage revision).1–57
With an acute infection (onset of less than 30 days) and a well-fixed implant, it is reasonable to attempt to retain the prosthesis by performing multiple debridements, typically every 2–4 days repeated at least three times.1 Joint debridement includes thorough irrigation, implant disarticulation, removal of necrotic tissue, polyethylene exchange and placement of antibiotic cement beads. Intraoperative cultures should be taken each time and antibiotic treatment directed by sensitivity results from these cultures. The patient should be treated with at least 6 weeks of intravenous/oral antibiotics. The effect of this treatment can be monitored by serial blood tests. If the infection persists, however, the prosthesis should be removed and replanted as a staged procedure when the infection is eradicated.
In a loose or chronically infected total elbow prosthesis, the implant should be removed and the joint debrided. All cement should be removed, using cortical windows if needed to gain access to the medullary canals and to remove well-fixed stems. Antibiotic laden cement spacers are employed. A second-stage reimplantation is performed once the infection has been eradicated following at least 6 weeks of parenteral antibiotic treatment. Although Gille et al5 have shown good results with single-stage revision using antibiotic-laden cement, single-stage prosthetic exchange is generally not recommended due to a high rate of recurrent infection.1 Excisional arthroplasty without reimplantation should be considered for persistent or recurrent infections, particularly when Staphylococcus epidermidis is the infecting organism.1 If a patient cannot tolerate multiple procedures due to poor soft tissue, excessive bone loss or medical comorbidities, excisional arthroplasty is also recommended (Fig. 44.1). Patients with excised elbow joints will often require bracing for symptomatic instability and comfort. While delayed reimplantation is an option, Cheung et al have reported that recurrence of infection remains high at 28%.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree