Fatigue is common in neuromuscular disease and it may affect quality of life; however, it has not been adequately studied. We can approach fatigue in neuromuscular diseases systematically. Questionnaires are used to assess subjective or experienced fatigue, and with the wide availability of the Internet, many patients can fill out questionnaires through Web-based surveys. Researchers can use force-generation protocols to evaluate physical fatigability and attention protocols to evaluate mental fatigability. Using these techniques to further understand the mechanisms of subjective and physiologic fatigue will help physicians to develop more effective treatments for fatigue and improve patients’ quality of life.
Neuromuscular diseases include a group of conditions that involve lower motor neurons (such as amyotrophic lateral sclerosis [ALS]), nerve roots (such as radiculopathy), plexuses (such as brachial plexopathy), peripheral nerves (such as axonal or demyelinating neuropathy), neuromuscular junctions (such as myasthenia gravis), and muscles (such as inflammatory myopathy). Some neuromuscular diseases can also involve upper motor neurons or the brain, however. For example, one of the diagnostic criteria for ALS is involvement of upper motor neurons. Patients with myotonic dystrophy have cognitive dysfunction and the severity of cognitive dysfunction correlates with the size of the CTG repeat.
Definition of fatigue
Fatigue is common in many medical and neurologic conditions. One of the major challenges in studying fatigue is the lack of a commonly accepted definition; both physicians and patients often talk about “fatigue” without explicitly defining the term. Harrison’s Principles of Internal Medicine describes chronic fatigue syndrome as “a disorder characterized by debilitating fatigue and several associated physical, constitutional, and neuropsychological complaints,” yet never defines fatigue. In practice, “fatigue” may have meanings ranging from mental depression to neuromuscular weakness. Establishing a working definition is the first step in assessing fatigue.
One obstacle in defining fatigue is that it is used to describe either a trait or a state. Whereas a trait is more or less chronic, a state is a relatively temporary condition. In the body of fatigue research, the term “subjective fatigue” or “experienced fatigue” usually refers to the general sensation of tiredness or of difficulty in initiating physical or mental activity over several days to weeks. This is often assessed by questionnaires completed by the subject. The term “fatigability” or “physiologic fatigue” refers to difficulty in maintaining the physical or mental activity at a desired level. Physicians are familiar with the fatigability test used to examine a patient who is suspected to have myasthenia gravis. In the fatigability test, the examiner asks the patient to contract a muscle (for example, the deltoid muscle) repetitively and evaluates whether or not the force generated declines after a few repetitions. The muscle tested is judged to be “fatigable” if the examiner detects a decline in the force generated. Fatigability occurs in a short period of time; therefore, it can be measured quantitatively in a laboratory setting. It is important to note that subjective fatigue and fatigability are not necessarily correlated. In other words, even if patients complain that they are “tired all the time,” they may perform well on measures of fatigability. Researchers need to be careful to correctly define and interpret findings of subjective fatigue and fatigability.
A second important differentiation is “physical” versus “mental” subjective fatigue and fatigability. Subjective physical fatigue refers to the amount of effort a subject feels he or she needs to complete certain physical activities, such as performing manual labor, walking, jogging, running, or lifting weights, which require skeletal muscles to generate force. Physical fatigability is the type of fatigability that is induced by motor tasks, such as force generation. Subjective mental fatigue refers to the effort subjects feel they must put forth to pay attention to tasks. Mental fatigability is the degree of attention a subject can maintain when required to sustain attention or concentration for a certain period of time. Subjective mental fatigue and physical fatigue are not always correlated with each other. To the best of the author’s knowledge, no studies have examined the correlation between mental and physical fatigability.
Systematic approach of fatigue in neuromuscular diseases
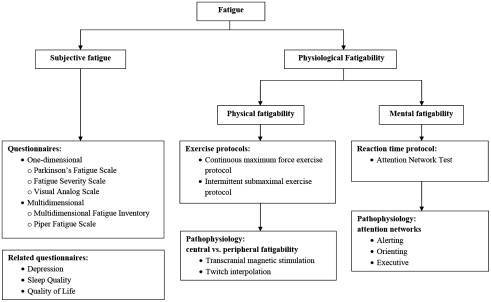
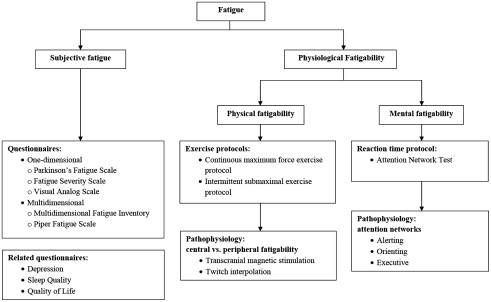
One of the best ways to assess fatigue in neuromuscular diseases is to take a systematic approach ( Fig. 1 ). We can assess subjective physical and mental fatigue using 1 dimensional and multidimensional questionnaires while assessing mental and physical fatigability in a laboratory setting. Researchers often use exercise protocols to evaluate physical fatigability and reaction time paradigms to evaluate mental fatigability.
Using Questionnaires to Assess Subjective Physical and Mental Fatigue
Both 1-dimensional and multidimensional questionnaires are valuable in assessing the presence, severity, and prevalence of subjective fatigue. One-dimensional instruments, such as the Visual Analog Scale (VAS) and 9-item Fatigue Severity Scale (FSS), give a single score to indicate the severity of fatigue. Multidimensional fatigue instruments, such as the Multidimensional Fatigue Inventory (MFI) and the Piper Fatigue Scale (PFS), contain several subscales usually based on a factor analysis. These 4 fatigue instruments have been used in other neurologic or neuromuscular diseases, such as multiple sclerosis, Parkinson disease (PD), ALS, and postpolio syndrome.
The Visual Analog Scale of fatigue
The VAS of fatigue is a 100-mm horizontal line representing the severity of fatigue ranging from 0 (right) to 100% (left). The subjects mark a point on the line that best represents the severity of their fatigue. The distance from the right is scored from 0 to 1.0. The scores in the Visual Analog Scale of fatigue correlated significantly with the multi-item fatigue subscale of the Profile of Mood States in subjects receiving chronic hemodialysis.
Not all of these questionnaires are suitable for all neuromuscular diseases. To choose a suitable questionnaire for a particular neuromuscular disease, researchers need to determine the reliability, ease of use, and construct validity of that fatigue questionnaire for measuring fatigue in that particular disease. In addition, scores of a questionnaire should independently predict quality of life when adjusted for severity of associated conditions, such as immobility, depression, sleepiness, pulmonary function, and pain.
The Fatigue Severity Scale
Krupp and colleagues developed this 1-dimensional, 9-item fatigue inventory and validated its internal consistency, sensitivity, and test-retest reliability. The 9-item FSS was selected from a 28-item questionnaire. The investigators administered the 28-item questionnaire to 25 subjects with multiple sclerosis, 29 subjects with systematic lupus erythematosus, and 20 healthy controls. They asked subjects to read each statement of the questionnaire and choose the number between 1 and 7 that best described their degree of agreement with each statement: 1 indicates strongly disagree and 7 strongly agree. Using factor analysis, item analysis, and theoretical considerations, they chose 9 items from this questionnaire to form the FSS. They found that the FSS has good internal consistency (with a Cronbach’s alpha of 0.89 for multiple sclerosis, 0.81 for systemic lupus erythromatosus, and 0.88 for healthy controls). They also examined sensitivity of the scale (ie, the ability of the scale to detect clinically appropriate and predicted changes in fatigue) by administering the scale to 6 subjects with Lyme disease before and after antibiotic treatment and 2 subjects with multiple sclerosis before and after treatment with pemoline, a stimulant. In all of these subjects, clinical improvement was associated with reduced scores. In addition, they examined the test-retest reliability of the scale by administering the scale to subjects in whom there was no clinical reason to expect changes in their fatigue state. The subjects were tested at 2 points of time separated by 5 to 33 weeks. As hypothesized, no significant changes in the scores were noted.
The Multidimensional Fatigue Inventory
The MFI is a 20-item self-report instrument designed to measure fatigue. The 20 items cover 5 dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity. Smets and colleagues tested the psychometric properties of the MFI in 111 cancer subjects receiving radiotherapy, 395 subjects with chronic fatigue syndrome, 481 psychology students, 158 medical students, 46 junior physicians, 160 army recruits during their stay in the barracks, and 156 army recruits in the second week of intensive training. They demonstrated that the MFI was well accepted in both general and clinical populations. Ninety-six percent of the respondents completed the MFI without omitting items. They determined the 5-dimensional structure using confirmatory factor analysis and demonstrated that the 5-factor model fit the data in all samples tested. The instrument had good internal consistency with a Cronbach’s alpha of 0.84. They also established the construct validity of the instrument by comparing groups, assuming differences in fatigue based on differences in circumstances or activity level. For example, subjects with chronic fatigue syndrome scored higher than students and army recruits in barracks. Army recruits scored higher during intensive training than when they were in barracks.
Researchers have used the MFI to assess fatigue in subjects with cancer and chronic obstructive pulmonary disease (COPD). In a study of subjects with COPD, Breslin and colleagues 14 measured pulmonary function, fatigue using the MFI, and depression using the Center for Epidemiologic Studies Depression Scale. They showed that depression correlated with general fatigue and mental fatigue but not with physical fatigue in the MFI. On the contrary, the severity of the pulmonary function impairment correlated with physical fatigue and reduced activity but not with mental fatigue. Other investigators have shown this separation between physical fatigue and mental fatigue as well.
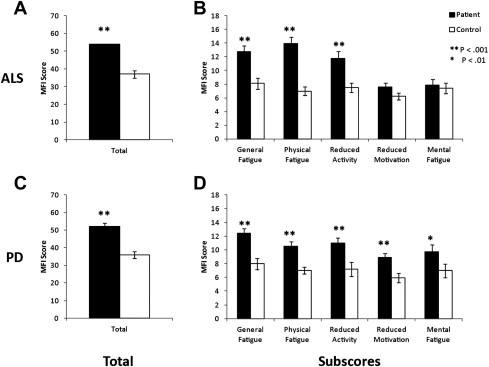
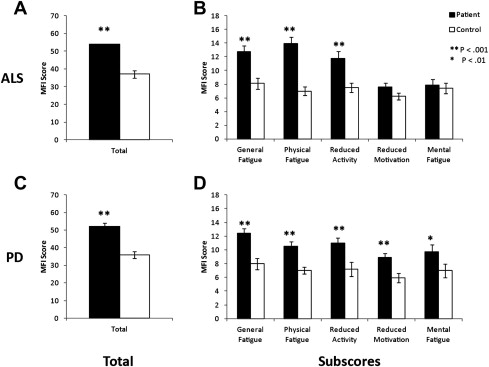
A multidimensional fatigue questionnaire, such as the MFI, is useful in characterize fatigue in different diseases. In studies in which the MFI was administered to patients with PD or ALS ( Fig. 2 ), a stark contrast in the distribution of MFI subsores emerged. Patients with PD or ALS had greater overall MFI scores than controls. The subscores revealed that patients with ALS do not show significantly higher fatigue in the “Reduced Motivation” and “Mental Fatigue” subscores than controls, whereas patients with PD display significantly higher fatigue in these categories than controls.
The Piper Fatigue Scale
The PFS is a multidimensional questionnaire and includes 22 characteristics of fatigue in 4 different dimensions: behavioral/severity, affective meaning, sensory, and cognitive/mood. The validity and reliability of the PFS have been well established in subjects with cancer, myocardial infarction, and HIV. The PFS has been validated in postpolio syndrome, a lower motor neuron disease. Strohschein and colleagues administered the PFS to 64 subjects with postpolio syndrome and 25 healthy controls. They demonstrated that the instrument has a high internal consistency with a Cronbach’s alpha coefficient of 0.98 and strong test-retest reliability with an intraclass correlation coefficient of 0.98. The convergent validity of the instrument was shown with a strong positive correlation between the PFS and Chalder Fatigue Questionnaire.
Subjects do not need to fill out these questionnaires in the presence of clinicians or researchers. Widely available Internet access is now making it possible for Web-based surveys to replace paper surveys. Many subjects can fill out these questionnaires online with minimum effort.
Measuring Physical Fatigability in a Laboratory Setting
Physical fatigability is the inability to maintain the desired force during sustained or repeated exercise. It can be assessed objectively is a laboratory setting. Two most commonly used exercise protocols are intermittent submaximal exercise protocol and continuous maximum force exercise protocol.
In the submaximal force protocol, the subject generates submaximal (usually 50% of MVC) contraction intermittently (eg, 3 to 5 repetitions per minute) ( Fig. 3 A). The submaximal force protocol mimics activities, such as walking or cycling, and fatigue develops over a longer period (10–30 minutes) than in the maximal force exercise protocol. Submaximal force generation is more common for activities of daily living and therefore is more relevant to study fatigue. In the submaximal exercise protocol, we first measure the baseline maximal voluntary contraction (BMVC) of the muscles of interest as the greatest MVC in 3 trials. We use the BMVC to calculate the 50% MVC to be used in the submaximal force protocol. For a duty cycle of 70%, the subject sustains a contraction of 50% MVC for 7 seconds and rests for 3 seconds repeatedly. After every 3 cycles, the subject attempts to perform an interval MVC (IMVC). This series is repeated until the subject is unable to generate an IMVC above 60% of the MVC. We use the slope of the IMVC to measure the development of physical fatigability.
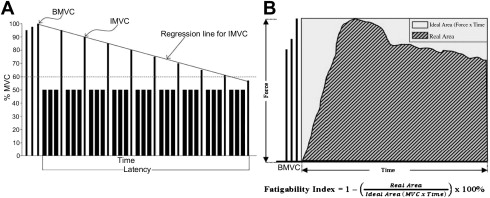
In the maximum force exercise protocol (see Fig. 3 B), the subject is instructed to generate sustained maximal voluntary contraction (MVC). The MVC is the force generated with the subject’s maximal effort with feedback and encouragement. During a sustained MVC, the force will decline gradually and fatigue will develop over a short period (<60 seconds). The maximal force protocol mimics activities, such as lifting heavy objects.
Systematic approach of fatigue in neuromuscular diseases
One of the best ways to assess fatigue in neuromuscular diseases is to take a systematic approach ( Fig. 1 ). We can assess subjective physical and mental fatigue using 1 dimensional and multidimensional questionnaires while assessing mental and physical fatigability in a laboratory setting. Researchers often use exercise protocols to evaluate physical fatigability and reaction time paradigms to evaluate mental fatigability.
Using Questionnaires to Assess Subjective Physical and Mental Fatigue
Both 1-dimensional and multidimensional questionnaires are valuable in assessing the presence, severity, and prevalence of subjective fatigue. One-dimensional instruments, such as the Visual Analog Scale (VAS) and 9-item Fatigue Severity Scale (FSS), give a single score to indicate the severity of fatigue. Multidimensional fatigue instruments, such as the Multidimensional Fatigue Inventory (MFI) and the Piper Fatigue Scale (PFS), contain several subscales usually based on a factor analysis. These 4 fatigue instruments have been used in other neurologic or neuromuscular diseases, such as multiple sclerosis, Parkinson disease (PD), ALS, and postpolio syndrome.
The Visual Analog Scale of fatigue
The VAS of fatigue is a 100-mm horizontal line representing the severity of fatigue ranging from 0 (right) to 100% (left). The subjects mark a point on the line that best represents the severity of their fatigue. The distance from the right is scored from 0 to 1.0. The scores in the Visual Analog Scale of fatigue correlated significantly with the multi-item fatigue subscale of the Profile of Mood States in subjects receiving chronic hemodialysis.
Not all of these questionnaires are suitable for all neuromuscular diseases. To choose a suitable questionnaire for a particular neuromuscular disease, researchers need to determine the reliability, ease of use, and construct validity of that fatigue questionnaire for measuring fatigue in that particular disease. In addition, scores of a questionnaire should independently predict quality of life when adjusted for severity of associated conditions, such as immobility, depression, sleepiness, pulmonary function, and pain.
The Fatigue Severity Scale
Krupp and colleagues developed this 1-dimensional, 9-item fatigue inventory and validated its internal consistency, sensitivity, and test-retest reliability. The 9-item FSS was selected from a 28-item questionnaire. The investigators administered the 28-item questionnaire to 25 subjects with multiple sclerosis, 29 subjects with systematic lupus erythematosus, and 20 healthy controls. They asked subjects to read each statement of the questionnaire and choose the number between 1 and 7 that best described their degree of agreement with each statement: 1 indicates strongly disagree and 7 strongly agree. Using factor analysis, item analysis, and theoretical considerations, they chose 9 items from this questionnaire to form the FSS. They found that the FSS has good internal consistency (with a Cronbach’s alpha of 0.89 for multiple sclerosis, 0.81 for systemic lupus erythromatosus, and 0.88 for healthy controls). They also examined sensitivity of the scale (ie, the ability of the scale to detect clinically appropriate and predicted changes in fatigue) by administering the scale to 6 subjects with Lyme disease before and after antibiotic treatment and 2 subjects with multiple sclerosis before and after treatment with pemoline, a stimulant. In all of these subjects, clinical improvement was associated with reduced scores. In addition, they examined the test-retest reliability of the scale by administering the scale to subjects in whom there was no clinical reason to expect changes in their fatigue state. The subjects were tested at 2 points of time separated by 5 to 33 weeks. As hypothesized, no significant changes in the scores were noted.
The Multidimensional Fatigue Inventory
The MFI is a 20-item self-report instrument designed to measure fatigue. The 20 items cover 5 dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity. Smets and colleagues tested the psychometric properties of the MFI in 111 cancer subjects receiving radiotherapy, 395 subjects with chronic fatigue syndrome, 481 psychology students, 158 medical students, 46 junior physicians, 160 army recruits during their stay in the barracks, and 156 army recruits in the second week of intensive training. They demonstrated that the MFI was well accepted in both general and clinical populations. Ninety-six percent of the respondents completed the MFI without omitting items. They determined the 5-dimensional structure using confirmatory factor analysis and demonstrated that the 5-factor model fit the data in all samples tested. The instrument had good internal consistency with a Cronbach’s alpha of 0.84. They also established the construct validity of the instrument by comparing groups, assuming differences in fatigue based on differences in circumstances or activity level. For example, subjects with chronic fatigue syndrome scored higher than students and army recruits in barracks. Army recruits scored higher during intensive training than when they were in barracks.
Researchers have used the MFI to assess fatigue in subjects with cancer and chronic obstructive pulmonary disease (COPD). In a study of subjects with COPD, Breslin and colleagues 14 measured pulmonary function, fatigue using the MFI, and depression using the Center for Epidemiologic Studies Depression Scale. They showed that depression correlated with general fatigue and mental fatigue but not with physical fatigue in the MFI. On the contrary, the severity of the pulmonary function impairment correlated with physical fatigue and reduced activity but not with mental fatigue. Other investigators have shown this separation between physical fatigue and mental fatigue as well.
A multidimensional fatigue questionnaire, such as the MFI, is useful in characterize fatigue in different diseases. In studies in which the MFI was administered to patients with PD or ALS ( Fig. 2 ), a stark contrast in the distribution of MFI subsores emerged. Patients with PD or ALS had greater overall MFI scores than controls. The subscores revealed that patients with ALS do not show significantly higher fatigue in the “Reduced Motivation” and “Mental Fatigue” subscores than controls, whereas patients with PD display significantly higher fatigue in these categories than controls.