Surgical Treatment of Overuse Injuries
Anthony A. Schepsis MD
Frederick J. Watson MD
William R. Creevy MD
Overuse injuries of the lower extremity are a commonly seen entity in the sports medicine clinic. Fortunately, only a handful of injured athletes present with a problem that requires surgical intervention to return to the field of play. In this chapter, we will focus on the surgical management of three specific entities—exertional compartment syndrome, anteriorly based “tension” tibial stress fractures, and stress fractures of the fifth metatarsal.
Exertional Compartment Syndrome
Historical Perspective
It wasn’t until it became clear that the exertional related pain that athletes would experience in the lower extremity could be a result of transient increases in intercompartmental pressure that it became intuitive that fascial release was the only surgical option. As far back as 1956, Mavor1 successfully treated anterior exertional compartment syndrome by a fascial widening procedure. Subsequently it was recognized that the extensile release necessary in acute compartment syndromes associated with trauma was not necessary and that only the investing fascia contributed to the symptoms in these athletic individuals. There was an evolution from the extensile single incision approach to two incisions with one specific for the anterior and lateral compartments and one specific for the superficial and deep posterior compartments that came into vogue. Many variations in technique have been described, including releasing the affected compartment from one incision, either large or small, or two smaller incisions. Recently endoscopic techniques that minimize morbidity have been introduced, as well as partial fasciectomy, in hopes of minimizing recurrence.
Indications and Contraindications
Exertional lower leg pain may be secondary to many causes; therefore, as in most orthopedic conditions, a careful history, physical examination, imaging studies, and compartment pressure studies are paramount in making the diagnosis before proceeding with operative intervention. Most commonly, the anterior and posterior compartments are involved; however, it is not unusual to have involvement of the lateral and superficial posterior compartments as well, usually in concert with involvement of the adjacent compartment. Most commonly it afflicts young athletes involved in running sports. The patient usually begins to experience a dull ache, crampiness, fullness, or a pressure sensation in the affected compartment that occurs after the individual has been exercising for a period of time. Although this period of time is variable from patient to patient, it usually has a predictable onset for that particular patient. For example, a long-distance runner usually notes that he or she can run at a certain speed and after a predictable period of time will have the onset of the symptoms. The pain usually begins as a dull ache or crampiness with a sensation of fullness. It is usually localized to the entire affected compartment. It usually increases to the point where activity has to be stopped. In some cases, the athlete may report an increase of intensity and duration with training or activity that pushes him or her over the threshold for generation of symptoms.
Sensory or motor disturbances sometimes occur, more commonly when the anterior compartment is affected. Transient “floppiness” of the ankle or a feeling of instability in the ankle and sensory disturbances in the distribution of the superficial peroneal nerve can occur when the anterior compartment is affected. This usually occurs more commonly when the athlete tries to “push through” the pain. In
many cases, as symptoms become more chronic, there is a quicker onset of symptoms as the individual meets the maximum tolerability in a shorter period of time. Typically, cessation of exercise will relieve the pain, with more severe pain diminishing over 10 to 15 minutes, but a dull, low-level of symptoms can continue for a significant period of time. Classically, the discomfort will virtually disappear until exercise once again commences. Bilaterality is more common than uncommon. Typically, the pain is felt in the soft tissues and not the bone, and there will be no persistent pain the next day. If the pain is more bony in origin and persists despite the cessation of exercise, stress fracture, or periostitis (medial tibial syndrome), should be considered.3
many cases, as symptoms become more chronic, there is a quicker onset of symptoms as the individual meets the maximum tolerability in a shorter period of time. Typically, cessation of exercise will relieve the pain, with more severe pain diminishing over 10 to 15 minutes, but a dull, low-level of symptoms can continue for a significant period of time. Classically, the discomfort will virtually disappear until exercise once again commences. Bilaterality is more common than uncommon. Typically, the pain is felt in the soft tissues and not the bone, and there will be no persistent pain the next day. If the pain is more bony in origin and persists despite the cessation of exercise, stress fracture, or periostitis (medial tibial syndrome), should be considered.3
Physical Examination
Unfortunately examination at rest is usually not helpful. Most of these patients are young, healthy athletes who are asymptomatic when they come into the office, making history of paramount importance in the suspicion of the diagnosis. In some cases, the athletes may be overconditioned with hypertrophy of the muscles and a compartment that cannot tolerate the 20% increase in muscle volume that is typically seen with exercise. It is important to look for fascial hernias, more typically seen in patients with anterior or lateral compartment syndrome. It has been reported in anywhere between 15% and 60% of patients. In the author’s (AAS) experience, approximately 15% to 20% of patients will have fascial hernias. Oftentimes these are not evident at rest and can only be seen with the patient standing on his or her heel with active dorsiflexion of the foot. This is typically seen at the junction of the mid and distal thirds of the leg, where the superficial peroneal nerve exits through the lateral intermuscular septum to become superficial. It is felt that most likely these fascial hernias represent an enlargement of the foramen through which the superficial peroneal nerve exits. Fascial hernias have a definite association with the development of exertional compartment symptoms.
It is important to examine the whole extremity, including alignment, range of motion, and stability of the knee and ankle, as well as postural foot abnormalities. Manual muscle testing usually reveals no weakness. Careful palpation of the bone is paramount to rule out a stress fracture. In the tibia, the most common location is at the junction of the proximal and mid thirds, with localized tenderness over the posteromedial border of the tibia. Less commonly, a more serious tibial stress fracture in the midshaft, in the anterior cortex, will lead to tenderness and bony fullness on the easily palpable mid and anterior tibial cortices. This more serious stress fracture will be discussed in the next section.
More commonly, patients who present with posterior or posteromedial exertional pain should be carefully examined for medial tibial syndrome.3 In these cases, there will be diffuse tenderness along the distal third of the posteromedial border of the tibia. This is considered to be a periostitis of the muscle attachments. Typically, these patients will also complain of symptoms with activities of daily living with persistent discomfort that gets worse with exercise and does not completely go away with rest. A careful examination of the distal pulses is important, although most of these athletes are young and do not have vascular claudication. The author (AAS) has seen several instances of dynamic popliteal artery entrapment being confused with exertional posterior compartment syndrome. Most commonly, patients will complain of coolness in the foot, but not always. Palpation of the posterior tibial pulse should be performed with the patient at rest and then with the patient actively plantar-flexing the foot with the knee extended. If the pulse diminishes with this maneuver, popliteal artery entrapment syndrome should be suspected and a vascular workup will be necessary. It is the author’s (AAS) practice when presented with a patient with exertional calf pain to perform noninvasive Doppler studies as a screening test to look at vascular flow within the posterior tibial artery at rest and then with active plantar flexion of the foot and calculate the ankle brachial indices. In the vast majority of cases, this will be negative; however, if this simple test is done, this entity will not be missed. In this entity, there is a dynamic compression of the popliteal artery as it goes under the soleus, causing transient claudication with exercise.4
It is the author’s (AAS) routine to examine the patient after reproduction of symptoms. This can be done either at the time of compartment pressure testing or by having the patient leave the office and run up and down stairs until the symptoms are reproduced. This will oftentimes help to substantiate the patient’s history and will help the physician to localize the symptoms, particularly if sensory disturbances and motor weakness transiently occurs.
In general, the pain with anterior exertional compartment syndrome tends to be well localized to the anterior compartment. Frequently, however, in a patient with exertional posterior compartment syndrome the pain may be very poorly localized. Therefore, it becomes even more important to rule out bony or vascular etiologies in these cases. Furthermore in patients with profound peroneal nerve weakness with marked weakness of dorsiflexion after exercise, peroneal nerve entrapment at the fibular head should also be suspected.2 The Tinel sign can usually be elicited in this location in these cases and the diagnosis can be confirmed by nerve conduction velocities of the peroneal nerve across the fibular head at rest and then after exercise.
After physical examination anteroposterior (AP) and lateral radiographs of the leg should be obtained to rule out any bony abnormalities. Chronic stress fractures can often be seen in these radiographs, but not always. It is the author’s (AAS) routine to perform a technetium bone scan in all of these patients, particularly if there is any bony tenderness, if they have posterior symptoms, or if they present
with a presumed medial tibial syndrome with diffuse tenderness along the posterior third of the distal tibia. In these cases, one would see increased uptake on the delayed images.
with a presumed medial tibial syndrome with diffuse tenderness along the posterior third of the distal tibia. In these cases, one would see increased uptake on the delayed images.
Compartment Pressure Testing
The key test in the diagnostic workup is the recording of intracompartmental pressure. Although the etiology can be multifactorial, it is generally agreed that transient ischemia at the capillary level is the responsible culprit for this symptom. During exercise, muscles only get blood flow during the relaxation phase of exercise. With muscle contraction, the pressure is much higher than the capillary perfusion pressure. Theoretically, testing the muscle relaxation pressure would be the most precise method to document muscle ischemia; however, this is not practical to the clinician. There is no role for dynamic measurement of pressures during exercise, since this is affected by the intensity of the muscle contraction as well as the depth of catheter insertion. Therefore, the most clinically useful measurements are to test the pressure before exercise, immediately after exercise, and then at 5 minutes after exercise. The most practical instrument is the slit catheter method. Although in our original clinical studies we used a continuous slit catheter measurement system,5 subsequently we have found that repeated needle insertion via a commercially available slit catheter (Stryker Corp, Kalamazoo, Mich) was more practical than an indwelling catheter that could move or clot off (Fig. 62-1).
Typically, we set up these pressure studies adjacent to a treadmill in the physical therapy department. By previous history and physical examination we have determined which compartments need to be tested. It is crucial that the catheter be inserted into the muscle and not the fascia or tendon. It is crucial to do all pressure measurements with the leg in a consistent position. We perform the pressure testing with the patient supine, with the foot in the neutral position, since it has been well established that foot position can affect the pressure measurements.6 For the anterior compartment, the catheter should be placed within the muscle belly of the tibialis anterior. The author’s (AAS) recommended landmark is the junction of the proximal and mid thirds of the leg, approximately 2 cm lateral to the tibial crest (Fig. 62-2). The skin and subcutaneous tissue are anesthetized with a longer acting anesthetic that will last the duration of the pressure testing. After calibration of the catheter, it is inserted through the fascia, which can be felt with a “pop” into the tibialis anterior muscle. Resting pressure
is recorded. For the lateral compartment, which is tested less commonly, the catheter is inserted at the same level, at the junction of the proximal and mid thirds of the leg, just over the fibular shaft in the muscle belly of the peroneals. For the superficial posterior compartment, placement of the catheter within the fleshy part of the medial head of the gastrocnemius is the easiest.
is recorded. For the lateral compartment, which is tested less commonly, the catheter is inserted at the same level, at the junction of the proximal and mid thirds of the leg, just over the fibular shaft in the muscle belly of the peroneals. For the superficial posterior compartment, placement of the catheter within the fleshy part of the medial head of the gastrocnemius is the easiest.
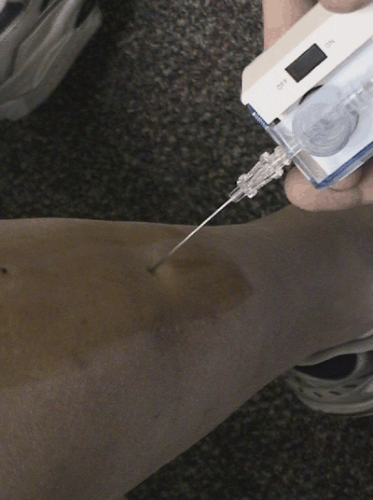 Fig. 62-2. Testing the anterior compartment is best performed by placing the needle in the muscle belly of the tibialis anterior in the proximal portion of the leg. |
Compartment pressure testing for the deep posterior compartment is the most difficult, being fraught with error and potential complications. The most volume of muscle is in the proximal portion of the deep posterior compartment; however, placing the catheter blindly in this location can be hazardous because of the adjacent blood vessels. Likewise, there are multiple subcompartments of the deep posterior compartment, particularly the tibialis posterior compartment.7 If one places a catheter in the distal part of the leg where the deep posterior compartment becomes more superficial, it is unfortunately not uncommon to place the catheter in or adjacent to tendon rather than muscle, making the pressure reading faulty. The author (AAS) usually recommends inserting the catheter carefully just posterior to the tibial shaft at the junction of the mid and distal thirds of the leg, at the location where the soleus bridge ends (Fig. 62-3). The catheter is carefully angled proximally and an initial pass is usually made with a 25-gauge needle to ensure that the vascular structures are not violated. If this trial is passed, the catheter needle is placed in this location, usually in the fleshy portion of the flexor digitorum muscle. Having the patient contract and relax the muscle will assure the examiner that the catheter is in the proper location. Some authors have recommended the use of ultrasound guidance for testing of the deep posterior compartment.8 Certainly if one would like to test the subcompartments, particularly the tibialis posterior compartment, computed tomography (CT) imaging or ultrasound guidance would be necessary. Davey et al.7 advocated testing this compartment by inserting the needle anteriorly through the interosseous membrane into the tibialis posterior. We have avoided this because of concerns about the safety of this maneuver. It has not been uncommon in the author’s (AAS) experience to have to repeat the deep compartment testing if there is any question as to its validity.
 Fig. 62-3. For testing the deep posterior compartment, the needle is inserted into the muscle belly of the FDL, just posterior to the posteromedial border of the tibia. |
After the resting pressures are obtained, the patient is instructed to run on the treadmill at an increasing cadence until symptoms are maximally reproduced. Usually placing the treadmill at an incline will help to reproduce symptoms faster. It is crucial that symptoms are maximally reproduced and the patient can run no longer before the postexercise pressure measurements are done. An immediate set of measurements after the cessation of exercise (1 minute) and then another set at 5 minutes are performed.
We feel that the pressure criteria set forth by Pedowitz et al.9 are the most useful. The presence of one or more of the following criteria would be consistent with a diagnosis of exertional compartment syndrome: a resting pressure of ≥15 mm Hg, a 1-minute postexercise pressure of ≥30 mm Hg, and/or a 5-minute postexercise pressure of ≥20 mm Hg.
In summary, the indications for surgical treatment would be a history that is consistent with the diagnosis, compartment pressure testing that is positive in at least one and preferably two out of the three criteria mentioned above, and a patient who is unwilling to modify his or her activity level. Again, it is important to consider all entities in the differential diagnosis, namely nerve entrapment, popliteal artery entrapment, vascular claudication, stress fractures, and medial tibial syndrome. Contraindications would include any vascular insufficiency to the lower extremity, extensive varicosities over the affected compartment, poor skin circulation, and a patient who is unwilling to accept the risks of any potential surgical complications or the potential for an unsuccessful outcome.
Surgical Technique
If the patient truly has exertional compartment syndrome, there really are only two choices: either modify his or her activity to alleviate the symptoms or have a surgical fasciotomy. Obviously the more competitive and avid the athlete, the more likely the patient is to undergo the latter choice. Although different surgical choices have been advocated, it is generally agreed that fasciotomy of the affected compartment is the treatment of choice for this condition. We prefer one incision to release the anterior and/or lateral compartments and one incision to release the deep and superficial posterior compartments of the leg. In those cases in which both the anterior and deep posterior compartments are involved, both incisions are utilized.
Surgical Release of the Anterior and Lateral Compartments
The technique of anterior compartment release involves making one skin incision to release both the anterior and
lateral compartments of the leg. The major structure at risk is the superficial peroneal nerve, which generally exits through the lateral intermuscular septum and becomes superficial at the junction of the mid and distal thirds of the leg, although there is significant anatomic variation. The patient is placed in the supine position on the operating table. Most of these patients are young and healthy and we prefer a light general aesthesia, usually a laryngeal mask anesthesia (LMA). An alternative is a short-acting spinal anesthesia, which is acceptable as well. A tourniquet is applied to the proximal thigh and we elevate the extremity approximately 3 to 4 minutes prior to exsanguinating the extremity with the use of an Esmarch bandage and inflating the tourniquet on the proximal thigh to anywhere between 250 to 300 mm Hg, depending upon the size and blood pressure of the patient. We divide the leg into thirds and mark the point at the junction of the mid and distal thirds of the lower leg. A 4- to 5-cm incision is made, with this point being the center point of the incision. The incision is located halfway between the tibial crest and the anterior border of the fibula. This is usually approximately 3 cm lateral to the tibial crest. We center the incision roughly over the lateral intermuscular septum, where the superficial peroneal nerve will be exiting. This is on average 10 cm proximal to the lateral malleolus (Fig. 62-4). In those cases where a fascial hernia is present, the incision should always be centered over the fascial hernia. The incision is carried down to the fascia and the fascia is exposed widely circumferentially. A transverse incision is made, starting at the tibial crest and carefully extending laterally to the intermuscular septum, looking closely for the superficial peroneal nerve (Fig. 62-5). The incision through the fascia is continued into the intermuscular septum to the posterior extent of the peroneal muscles. Retractors are placed in the superior and inferior fascia and then the fascia is retracted and the superficial peroneal nerve is identified.
lateral compartments of the leg. The major structure at risk is the superficial peroneal nerve, which generally exits through the lateral intermuscular septum and becomes superficial at the junction of the mid and distal thirds of the leg, although there is significant anatomic variation. The patient is placed in the supine position on the operating table. Most of these patients are young and healthy and we prefer a light general aesthesia, usually a laryngeal mask anesthesia (LMA). An alternative is a short-acting spinal anesthesia, which is acceptable as well. A tourniquet is applied to the proximal thigh and we elevate the extremity approximately 3 to 4 minutes prior to exsanguinating the extremity with the use of an Esmarch bandage and inflating the tourniquet on the proximal thigh to anywhere between 250 to 300 mm Hg, depending upon the size and blood pressure of the patient. We divide the leg into thirds and mark the point at the junction of the mid and distal thirds of the lower leg. A 4- to 5-cm incision is made, with this point being the center point of the incision. The incision is located halfway between the tibial crest and the anterior border of the fibula. This is usually approximately 3 cm lateral to the tibial crest. We center the incision roughly over the lateral intermuscular septum, where the superficial peroneal nerve will be exiting. This is on average 10 cm proximal to the lateral malleolus (Fig. 62-4). In those cases where a fascial hernia is present, the incision should always be centered over the fascial hernia. The incision is carried down to the fascia and the fascia is exposed widely circumferentially. A transverse incision is made, starting at the tibial crest and carefully extending laterally to the intermuscular septum, looking closely for the superficial peroneal nerve (Fig. 62-5). The incision through the fascia is continued into the intermuscular septum to the posterior extent of the peroneal muscles. Retractors are placed in the superior and inferior fascia and then the fascia is retracted and the superficial peroneal nerve is identified.
 Fig. 62-5. After identification of the nerve, a transverse fascial incision is made from the tibial crest, across the septum, to the personal muscles. |
Since superficial peroneal nerve entrapment is sometimes part of the symptom complex with anterior and lateral compartment syndrome, the superficial peroneal nerve should always be identified and explored proximally and distally into its branches. Furthermore, by identifying the nerve, iatrogenic injury to the nerve can be avoided as well. Fasciotomy of the anterior compartment is then performed approximately 1 cm lateral to the tibial crest. Long fasciotomy scissors are utilized (Fig. 62-6). Narrow, but deep right angle retractors are utilized to retract the subcutaneous tissues from the fascia. Extensive time should be taken to bluntly spread all of the soft tissue away from the
fascia, both on its anterior as well as posterior surfaces. After ensuring that the fascia is freed of any extraneous tissue, the fascia is first split going proximally. In the anterior compartment, this is the approximately 1 cm lateral to the crest, with the tips of the scissors pointing toward the crest (Fig. 62-7). In very large patients, it may be advisable to make a small 2-cm counterincision proximally in order to adequately complete the fasciotomy proximally. In small, thinner patients, however, this is not necessary. The fasciotomy is then completed going distally, again keeping the points of the scissors pointing to the tip of the crest.
fascia, both on its anterior as well as posterior surfaces. After ensuring that the fascia is freed of any extraneous tissue, the fascia is first split going proximally. In the anterior compartment, this is the approximately 1 cm lateral to the crest, with the tips of the scissors pointing toward the crest (Fig. 62-7). In very large patients, it may be advisable to make a small 2-cm counterincision proximally in order to adequately complete the fasciotomy proximally. In small, thinner patients, however, this is not necessary. The fasciotomy is then completed going distally, again keeping the points of the scissors pointing to the tip of the crest.
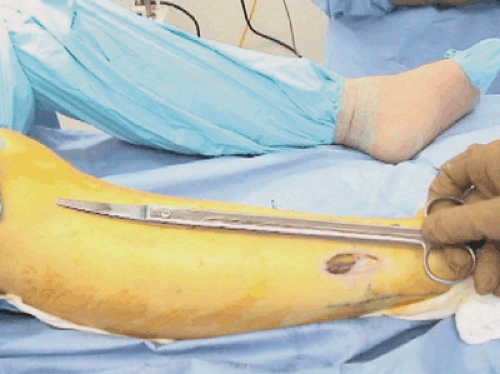 Fig. 62-6. These long curved fasciotomy scissors allow access to the most proximal extent of the compartment. |
The lateral compartment fasciotomy (if indicated) is performed in a similar fashion at the most posterior extent of the transverse fascial incision. The fasciotomy should be done as lateral as possible, again to avoid any branches of the superficial peroneal nerve distally, which have already been identified. In this case, the fascial release should be 1 to 2 cm anterior to the fibula with the tips of the fasciotomy scissors pointing laterally and posteriorly away from the superficial peroneal nerve (Fig. 62-8).
The tourniquet is deflated and hemostasis achieved using electrocautery. It is it important to identify any bleeders and obtain hemostasis even if this requires extending the incision. Marked bleeding can lead to extensive fibrosis and failure of the procedure. Recurrences are more related to thick fibro proliferative scar that forms around the incision and can generate local high pressures and entrap the superficial peroneal nerve.10 Therefore, the use of suture material should be minimized. The subcutaneous tissue is closed with interrupted 2.0 absorbable suture and the skin is routinely closed with a subcuticular nonabsorbable suture that can be removed 2 weeks postoperatively. A large compressive dressing is applied with large ABD pads over the whole extent of the compartments that have been released, with elastic bandages from toes to knee. Immediate postoperative care includes ice and elevation and ambulation with crutches.
Surgical Release of the Posterior Compartments
After similar preparation and anesthesia as described for the anterior compartment, a vertical 6-cm incision is made 2 cm posterior to the posteromedial border of the tibia. This incision is centered at the junction of the mid and distal thirds of the lower leg. This area is rich in vascularity and care must be taken to identify, protect, or ligate all of the small venous communicators in this area. Similar to the anterior release, a transverse incision is made going from the posteromedial border of the tibia and crossing the septum, separating the deep and superficial posterior compartments. Care must be taken to identify the saphenous nerve and vein as well as any communicators in this area. The potential for injury to the venous system is the most likely adverse event.
The superficial fascia is first split at the posterior part of the wound, extending proximally and distally with the tips pointed posteriorly, with a similar technique that is used for the anterior compartment. Both gastrocnemius muscles, however, have separate compartments (a posterior superficial medial and lateral) and if there is a compartment syndrome definitely involving the heads of the gastroc, a separate small incision may be necessary to release the fascia over the lateral head of the gastrocnemius.
Release of the deep posterior compartment, particularly proximally, is the most difficult and hazardous of any of the fascial releases, with the most common sequelae being deep venous bleeding. The deep fascia is identified in the distal third of the leg where the deep posterior compartment
becomes superficial distal to the end of the soleus bridge. The fascia usually can be easily divided going distally. Proximally, however, it is crucial to have long retractors lifting up the soleus bridge and to free up all of the soft tissue off of the fascia. Good visualization is paramount and this should not be done blindly. The distal 2 to 3 cm of the soleus bridge is detached in order to more completely expose the fascia over the deep posterior compartment, covering the flexor digitorum longus (FDL) (Fig. 62-9). The fascia covering the FDL is incised through its length going proximally, again being cognizant of the location of the saphenous nerve and vein. Deeper dissection to expose and release the subcompartment over the posterior tibial muscle is more hazardous and controversial. Retraction of the FDL allows inspection of the tibialis posterior compartment. If the fascia appears thickened and constricting, it is released within the limits that the incision allows. Blind fasciotomy proximally of this compartment is hazardous and not recommended and if necessary requires a more extensile approach. It would necessitate a larger incision with extensive detachment of the soleus bridge. The morbidity of performing this maneuver may outweigh potential benefits.
becomes superficial distal to the end of the soleus bridge. The fascia usually can be easily divided going distally. Proximally, however, it is crucial to have long retractors lifting up the soleus bridge and to free up all of the soft tissue off of the fascia. Good visualization is paramount and this should not be done blindly. The distal 2 to 3 cm of the soleus bridge is detached in order to more completely expose the fascia over the deep posterior compartment, covering the flexor digitorum longus (FDL) (Fig. 62-9). The fascia covering the FDL is incised through its length going proximally, again being cognizant of the location of the saphenous nerve and vein. Deeper dissection to expose and release the subcompartment over the posterior tibial muscle is more hazardous and controversial. Retraction of the FDL allows inspection of the tibialis posterior compartment. If the fascia appears thickened and constricting, it is released within the limits that the incision allows. Blind fasciotomy proximally of this compartment is hazardous and not recommended and if necessary requires a more extensile approach. It would necessitate a larger incision with extensive detachment of the soleus bridge. The morbidity of performing this maneuver may outweigh potential benefits.
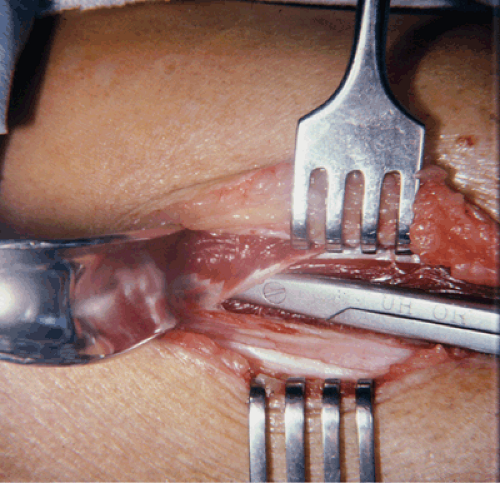 Fig. 62-9. Mobilization and retraction of the soleus bridge is mandatory to expose the fascia over the deep posterior compartment. |
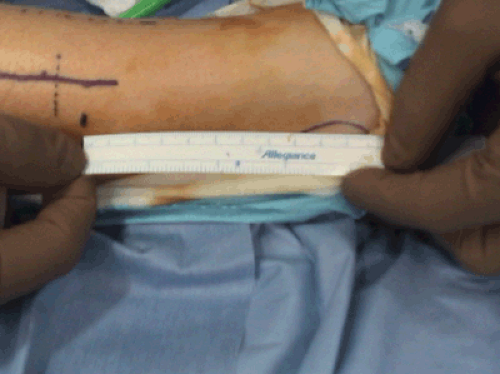
In bilateral cases, when one side is completed, the tourniquet is deflated and a temporary compressive wrap is placed on this extremity while addressing the other extremity. We usually recommend using a ruler to mark exactly the location of the incision, so that it can be placed symmetrically on the other extremity for cosmetic reasons. After the second extremity is addressed, a temporary compressive dressing is placed and the first extremity is reexamined for any bleeding points and a routine closing is performed. Then, the second extremity is addressed in a similar fashion.
Technical Alternatives and Pitfalls
Leversedge et al.11 described a one- and two-incision arthroscopically assisted fasciotomy, utilized not only to minimize the size of the incision, but also to minimize the amount of postoperative scar that forms, potentially decreasing the chance of recurrence. However, it may be difficult to evaluate and treat any concomitant nerve entrapment with this technique. Early reports have been promising; however, more experience with this technique is necessary before we would recommend this.
An alternative technique describes doing a partial fasciectomy rather than fasciotomy, removing a 1-cm strip of fascia rather than slitting the fascia, hopefully to prevent regrowth of the fascia and reoccurrence of symptoms. In one large series, the overall success rate was no better with this technique than with standard techniques.12 Interestingly, the results with this technique were reported to be better in the posterior compartment than in the anterior compartment. In a recent study the authors did a series of revision cases, where partial fasciectomy was utilized and they reserved fasciectomy for revision surgery.10
The main pitfalls occur around neurovascular injury. At risk anteriorly is the superficial peroneal nerve. Posteriorly, however, there is a rich vascular network. Bleeding issues are much more common, both venous as well as arterial. Superficially, the saphenous nerve and vein are at risk. Deeply, the major vessels are at risk. This procedure should never be done blindly and the operator has to be ensured that all soft tissue is away from the fascia when it is incised. There is never any harm in extending the incision to ensure visualization. It is critical to achieve hemostasis at the end of the procedure and to recognize any bleeding vessels prior to closure.
Postoperative Management
In unilateral cases, we recommend non–weight bearing for approximately a week, just to control swelling. In bilateral cases, weight bearing is allowed as tolerated with the use of two crutches for the first week and then gradual discontinuation of the crutches during the second week. Immediate range of motion exercises for the knee and ankle are prescribed. A compressive dressing is maintained for the first 3 to 4 days. After the sutures are removed at the 10- to 14-day mark, stretching exercises and isometric strengthening exercises are prescribed. Stationary bicycling is allowed at 2 weeks postoperatively, but jogging or running is not allowed until 8 to 12 weeks postoperatively. In general, patients with isolated anterior compartment fasciotomies have the quickest rehabilitation, and usually these patients
can resume running within 6 to 8 weeks after surgery. Patients who had deep posterior compartment release or 4-compartment release usually require at least 12 weeks of rehabilitation before running is permitted.
can resume running within 6 to 8 weeks after surgery. Patients who had deep posterior compartment release or 4-compartment release usually require at least 12 weeks of rehabilitation before running is permitted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree