CHAPTER 61 Surgical Decompression for Foraminal Stenosis
ETIOLOGY OF FORAMINAL STENOSIS
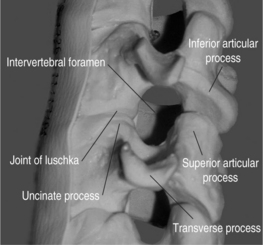
The cervical functional spinal unit consists of two vertebral bodies joined by an intervertebral disc anteriorly and two synovial facet joints posteriorly. The cranial surface of the vertebral body is typically concave from side-to-side and convex in the anteroposterior direction. The inferior surface is convex from side-to-side and concave in the anteroposterior direction. A projection on the lateral aspect of the superior surface of the inferior vertebral body is called the uncus. The uncus is intimately related to the convex lateral inferior surface of the cephalad vertebrae. This projection off the cephalad vertebral body is referred to as the enchanure or anvil. The ‘articulation’ between these two structures is referred to as the ‘uncovertebral joint’ or ‘joint of Luschka.’ Cervical nerve roots exit the spinal canal through the neural foramen in an anterolateral and inferior direction. The boundaries of the neural foramen are formed superiorly and inferiorly by the adjacent pedicles. The medial aspect of the facet joint and an adjacent portion of the articular column form the posterolateral border of the neural foramen. The anterolateral border is formed by the posterolateral portion of the uncus, the intervertebral disc, and the inferior portion of the superior adjacent vertebrae. Figure 61.1 shows a cervical spine model, viewed in an oblique projection, which illustrates the bony confines of the neural foramen.
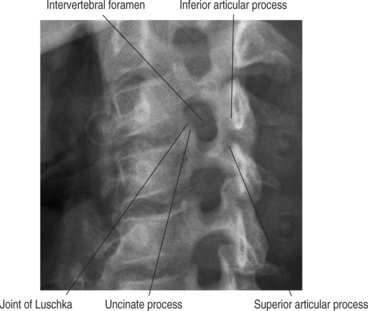
Neural foraminal narrowing can occur as a result of both static and dynamic processes. Abnormal biomechanics that result from biochemical changes in the intervertebral disc may lead to microinstability that contributes to dynamic neural foraminal narrowing. Changes in the bony architecture of the borders of the foramen lead to static narrowing of the neural foramen. Posteriorly protruding disc material, uncovertebral osteophytes, or thickened soft tissues within the foramen may result in extrinsic compression of the nerve root. An oblique radiograph of the cervical spine illustrates the anatomic borders of the foramen and how uncovertebral joint osteophytes can result in narrowing of the foramen. Both Figure 61.1 and Figure 61.2 demonstrate how osteophyte formation from the facet joint could result in narrowing in the anterior–posterior dimension. The height of the foramen may also be compromised as the intervertebral disc loses its biomechanical integrity and loses height. As mentioned earlier, the vertebral bodies move closer together, causing the adjacent pedicles to move closer, thereby narrowing the cephalad–caudad dimension of the neural foramen. Surgical treatment of neural foraminal stenosis must address the anatomic structures that are responsible for narrowing of the foramen and may include an arthrodesis to eliminate any dynamic foraminal narrowing.
CLINICAL PRESENTATION AND PATIENT EVALUATION
Foraminal stenosis typically results in cervical radiculopathy which refers to symptoms that occur in a specific dermatomal pattern in the upper extremity. Patients typically describe sharp pain and tingling or burning sensations in the involved area. Diminished sensation and motor loss corresponding to the involved nerve root may be present, as may diminished deep tendon reflexes. Patients typically have neck and arm pain that prevents them from finding a comfortable position. They may find comfort by placing their forearm on top of their head, the so-called ‘shoulder abduction sign.’1 Extension or lateral rotation of the head to the side of the pain (Spurling’s maneuver) may aggravate the symptoms. This finding may assist in the differentiation of radicular pain from other sources of shoulder pain and muscular neck pain. It is also paramount to remember that multiple sources of neck and upper extremity pain are extremely common and that neural compression may very well be occurring at two distinct locations.2,3 Adaptations to the initial radiculopathy symptoms may result in secondary pathologic changes in the shoulder, carpal tunnel, and ulnar nerve that may cloud the clinical picture and persist long after the cervical radiculopathy has resolved. In a series of 736 patients with cervical radiculopathy treated operatively, Henderson et al. reported the clinical signs and symptoms at presentation. They found that 99.4% had arm pain, 85.2% had sensory deficits, 79.7% had neck pain, 71.2% had diminished deep tendon reflexes, 68% had motor weakness, 52.5% had periscapular pain, 17.8% had anterior chest pain, and 9.7% had headaches. The neurologic deficits at presentation correlated with the offending disc level in approximately 80% of the patients.4
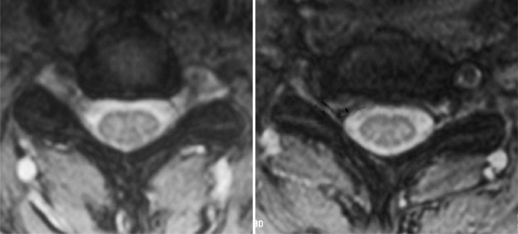
Additional studies that should be considered include bone scan, myelography combined with computed tomography (CT), electrodiagnostic testing, and magnetic resonance imaging (MRI). Consideration for bone scans should be given in patients with suspected malignancy, infection, or occult trauma realizing that bone scan may be normal in the setting of multiple myeloma. Myelography combined with computed tomography was the study of choice for the evaluation of suspected cervical stenosis until the widespread availability of MRI. Myelo/CT studies are still useful in those patients with contraindications to MRI and in potential revision situations. MRI has become the gold standard imaging modality for evaluation of patients with cervical radiculopathy and myelopathy. The advantages of MRI are that it provides complete visualization of the vertebrae, intervertebral discs, nerve roots, subarachnoid space, and spinal cord, including any intraspinal cord pathology. Multiplanar images are also routinely generated by this noninvasive, readily available imaging modality. It provides exquisite sensitivity in detecting subtle anatomic abnormalities. Axial images at the level of the neural foramen provide excellent contrast between the cerebrospinal fluid (CSF)-filled nerve root sleeve and the surrounding bony and soft tissue structures. Foraminal narrowing from disc herniation, uncovertebral osteophyte, facet hypertrophy, and in-folded ligamentum flavum can be visualized on these axial T2 images. An example of foraminal stenosis caused by uncovertebral osteophytes can be seen in Figure 61.3.
SURGICAL DECOMPRESSION OF FORAMINAL STENOSIS
Patients presenting with symptoms of cervical radiculopathy and imaging studies that coincide with the physical exam findings should be considered for active treatment. Medical rehabilitation and interventional spine treatment modalities including nonsteroidal antiinflammatories, mild narcotic analgesic medications, physical therapy, cervical epidural steroid injections, and selective nerve root blocks should be utilized initially for most patients. Absolute indications for surgical decompression of isolated foraminal stenosis are lacking although patients presenting radicular symptoms in combination with progressive myelopathy should be treated surgically. Strong indications for surgical intervention include profound muscle weakness and atrophy. Surgical decompression should also be considered if a patient has undergone a prolonged period of conservative management without resolution of their symptoms. A trial of 6–12 weeks of nonoperative modalities is considered adequate by most surgeons. The severity of the patient’s pain and the degree of muscle weakness play a role in the decision to abort conservative treatment and proceed with surgical intervention.
ANTERIOR CERVICAL DISCECTOMY AND FUSION
The anterior approach to the cervical spine allows for direct access to the compressive pathology responsible for foraminal stenosis. Bulging or herniated disc material and uncovertebral osteophytes lie anterior to the spinal cord and the nerve roots and can therefore be directly removed from an anterior approach. Figure 61.4 shows how these offending anterior structures are directly accessible for removal via an anterior approach. Robinson and Smith initially described anterior cervical discectomy and fusion in 1955.5 The anterior approach avoids direct manipulation of the neural elements. This original procedure relied on indirect decompression of the spinal cord and neural foramen by distraction of the disc space. Progression of spondylotic spur formation was prevented by stabilization of the segment and the authors believed that regression of these osteophytes occurred as a result of the arthrodesis. In 1958, Cloward published an alternative technique for anterior cervical discectomy and fusion in which direct decompression of the neural elements was performed.6 There are no comparative studies in the literature to support the clinical superiority of direct or indirect decompression of the neural elements in spondylotic radiculopathy. Individual surgeons must make a decision as to which philosophy they subscribe. The surgeon’s training as well as personal experience likely influence this decision.
Numerous modifications of the original Smith–Robinson and Cloward bone grafting techniques have been described. The Smith–Robinson technique involves an autogenous tricortical corticocancellous horseshoe graft placed into the evacuated disc space. The cortical component of the graft provides structural support that maintains the disc-space height while the cancellous portion facilitates graft incorporation. The original technique involved simply removing the cartilaginous endplate and incomplete decortication. More aggressive decortication, beyond simple cartilaginous endplate removal, has been demonstrated to improve fusion rates.7,8 Cloward’s autogenous graft was harvested from the iliac crest with an oversized hole saw. The oversized dowel graft was then impacted into an undersized hole at the level to be fused. The unicortical surface of this dowel graft was placed anteriorly, resting just posterior to the anterior surface of the vertebral bodies. Bailey and Badgley described a technique in which a ‘keystone-shaped’ corticocancellous graft was utilized.9 This trapezoidal-shaped graft is mortised into the prepared disc space. Excellent results with this technique were reported by Simmons in 1969.10
Contemporary choices for graft material include autograft, allograft, and synthetic cage devices. Good results have been reported with all of these graft choices when utilized for one-level discectomy and fusion without instrumentation, although the current literature does not support the superiority of any one single graft material or device.7,11–15 Higher rates of radiographic arthrodesis have been reported with the use of autogenous iliac crest grafts, especially with multiple-level discectomies.12,16 Higher radiographic fusion rates with autogenous grafts do not occur without consequences. Complications related to graft harvest include hematoma formation, infection, injury to the lateral femoral cutaneous nerve, iliac wing fractures, and, most importantly, persistent iliac crest pain have been reported.17,18 Silber et al. reported iliac crest graft site harvest complications in a series of patients undergoing one-level anterior cervical discectomy and fusion (ACDF). Twenty-six percent of patients reported chronic pain at the donor site and nearly half of these chronically used pain medications. They also report functional impairment in 13% of the patients studied.17
Allografts have been used in anterior cervical spine surgery since the 1950s. Cloward reported a 94% fusion rate in 44 patients who underwent ACDF with a dowel-shaped, frozen allogeneic iliac crest graft.6 Schneider utilized freeze-dried allogeneic iliac crest bone for ACDF with the Cloward technique. He reported an arthrodesis rate of 93% with one-level procedures and an 85% fusion rate for multiple-level discectomy and fusion.19 The use of allograft iliac crest grafts with the Smith–Robinson has also been reported in the literature. Rish reported a fusion rate of 80% in single-level and 69% in multiple-level patients undergoing a Smith–Robinson-type ACDF with freeze-dried allogeneic iliac crest.20 A 94% fusion rate was reported by Brown et al. in patients undergoing the same procedure with allogeneic, frozen iliac crest grafts.21 They also reported a high rate of graft collapse when allogeneic iliac crests are used. As a result of concerns over the compressive strength of allograft iliac crest grafts, some authors began utilizing fibular allograft for anterior cervical discectomy and fusion. Martin et al. reported on 289 patients who underwent ACDF with freeze-dried fibular allograft without instrumentation.11 Radiographic fusion was obtained in 90% of those undergoing one-level fusions. After two-level procedures, only 72% showed radiographic fusion at all levels. Although there was a trend towards higher rates of nonunion in those patients who were smokers, the difference was not statistically significant. The authors concluded that freeze-dried allogeneic fibula is an effective substrate for obtaining fusion following anterior cervical discectomy without instrumentation.
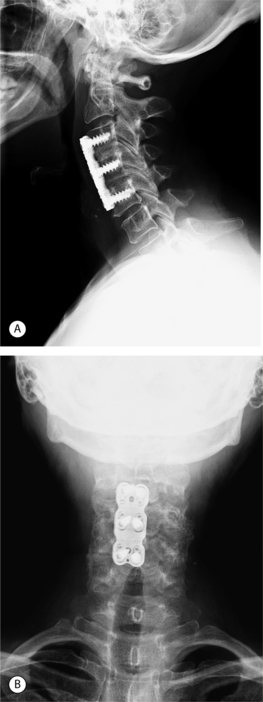
In an attempt to increase the likelihood of solid arthrodesis, many authors have advocated the addition of an anterior cervical plate, especially for multiple-level procedures. Radiographs of a two-level anterior discectomy and fusion with an anterior cervical plate are shown in Figure 61.5. Instrumentation increases the stability across the operative segment and decreases the motion between the graft and vertebral endplate, thereby increasing the chance of solid arthrodesis. The addition of anterior cervical instrumentation has been shown to decrease the rate of pseudoarthrosis in both single and multiple-level ACDFs. Nonunion rates ranging 11–63% have been reported depending on the number of levels at attempted fusion, the type of graft material used, and whether or not anterior instrumentation was utilized.12,22–25
Wang and colleagues reviewed the clinical and radiographic results of 80 patients treated with single-level ACDF.26 Pseudoarthrosis rates of 4.5% and 8.3% were reported for instrumented and noninstrumented, respectively. Patients stabilized with an anterior plate had significantly less graft collapse and kyphosis than those without a plate. The clinical outcomes based on Odom’s criteria were not significantly different between the two groups. In two separate reports, the same senior authors have also reported retrospectively on the use of anterior cervical plates in two- and three-level ACDFs.27,28 Autogenous tricortical iliac crest grafts were used in all patients in each study. In the review of two-level procedures, a statistically significant difference in pseudoarthrosis rate was found in the instrumented group (0%) compared to the noninstrumented group (7%).27 The use of a cervical plate did not alter clinical outcomes but those patients who developed a pseudoarthrosis had poorer clinical results. In the third report by this group, the results of three-level ACDF were reviewed.28 Forty of the fifty-nine patients were stabilized with an anterior cervical plate. Pseudoarthrosis developed in 18% (7/40) of the plated patients and 37% (7/19) of those treated without a plate. This difference was not statistically significant, perhaps because of the small number of patients, but the authors point out that the rate of nonunion in the noninstrumented group is nearly twice that of the instrumented group. Once again, the development of a pseudoarthrosis resulted in poorer clinical outcomes.
Other groups have analyzed the results of both one- and two-level ACDFs treated with and without plates.29,30 Caspar et al. retrospectively reviewed the charts of 356 patients who underwent one- and two-level ACDF with and without anterior instrumentation for degenerative disorders.30 Six percent of those undergoing noninstrumented fusions required reoperation for pseudoarthrosis compared to 0.7% in whom an anterior cervical plate was used. Two (out of 146) patients in whom a plate was used required a revision procedure secondary to hardware failure prior to fusion. The authors concluded that the use of anterior instrumentation for one- and two-level ACDFs results in a significant reduction in reoperation rates. In a retrospective manner, Kaiser et al. compared the radiographic results of a group of patients stabilized with anterior cervical plates following one- and two-level ACDF with a historical cohort of patients that were treated without plates.29 Cortical ring allograft struts were utilized in each of the groups. The fusion rates for one- and two-level ACDF with anterior instrumentation were 96% and 91%, respectively. Significantly lower fusion rates were found in those one- and two-level procedures (90% and 72%, respectively) that were not stabilized by an anterior plate.11 No infectious, neurologic, or graft-related complications were reported in the instrumented group. Statistical analysis revealed a higher graft complication rate in the noninstrumented group from the historical cohort. The authors conclude that the improved fusion rates and negligible complication rates justify the routine use of anterior cervical plating in the treatment of cervical spondylosis.
Surgical technique
Any remaining anterior osteophytes are removed with a rongeur or high-speed burr. This will allow the cervical plate to lie flat against the anterior aspect of the spine, thereby avoiding any possible issues with dysphagia from the instrumentation. Failure to completely remove this abnormal bone also results in the plate resting on the osteophytes with the screws obtaining some of their purchase in this soft bone. The distraction across the disc space is released and the distraction pins are removed. To provide additional compression of the graft, the neck is brought out of extension by placing a towel behind the occiput. The authors’ choice of plating systems is a slotted, dynamic plate that allows for both dynamic compression of the graft as the screws are tightened, and linear settling of the graft as the cranial screws slide through the slots in the plate. During the insertion of the plate care must be taken to assure that the cranial screws are entering the vertebral body at the graft–host bone junction. Failing to do so may result in damage to the superior adjacent segment as the operative level settles and the cranial edge of the plate approaches the disc space proximal to the fusion. This is more of a concern with multiple-level discectomies but should not be neglected with one-level fusions. An appropriate length of plate is placed flat against the anterior aspect of the spine. The drill holes are drilled for the inferior screws first with a drill bit with a stop at the appropriate length. The length of these screws is determined by the overall size of the patient and by preoperative radiographs. Alternatively, the depth of the vertebral body may be determined intraoperatively after the discectomy and decompression have been completed. Unicortical screws typically range 12–16 mm in length. The drill guide for the inferior screws is a ‘static’ guide, which places the screw in the center of the hole in the plate. The two caudal screws are placed but not tightened securely to the plate, thereby avoiding tipping the superior end of the plate anteriorly. The guide for the cranial screws is a ‘dynamic’ drill guide, which preferentially drills the guide hole eccentrically in the slot of the plate. The superior screws are placed but are also not secured tightly to the plate. Once all four screws have been placed, the inferior, or ‘static,’ screws are fully tightened. As the ‘dynamic’ screws at the top of the slots at the cranial end of the plate are tightened, a compressive force is place across the graft. Lastly, the screw-locking mechanism is engaged to prevent back-out of the screws. A lateral radiograph is obtained to confirm the position of the plate, the maintenance or recreation of the cervical lordosis, and the position of the screws. The self-retaining retractor is removed and sidewalls of the wound are inspected for any bleeding. The wound is irrigated with antibiotic saline and a small Jackson-Pratt drain placed directly over the plate and passed out the superior or lateral aspect of the wound. The platysma is closed with absorbable sutures followed by a running subcuticular closure of the skin. Because of the used of routine anterior cervical plating, all single-level discectomies are placed into a soft cervical collar that is worn for comfort only. Multilevel discectomies and corpectomies are placed into a semirigid cervical orthosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree