Staged Revision of the Infected Total Knee Arthroplasty
Jess H. Lonner
Javad Parvizi
INDICATIONS/CONTRAINDICATIONS
Despite all attempts for prevention, periprosthetic joint infection continues to pose a great clinical problem, and its incidence may be on the rise (1, 2, 3). In fact, periprosthetic infection may now be the main cause of failure of total knee arthroplasty (TKA). The challenges of treating this complication relate to difficulty in diagnosis in some patients and selection of the most appropriate treatment modality. The main objective of the treatment is to eradicate infection and provide a functioning and pain-free joint for affected patients. The latter, however, can be difficult to accomplish in some cases.
We use a simple classification scheme that helps define an appropriate treatment algorithm for the infected TKA. Simplistically, this scheme distinguishes between acute and late infections. Acute perioperative infections generally occur within 3 weeks of surgery and can occasionally be treated effectively with thorough open debridement and lavage with retention of the implants, depending on the organism. Success with irrigation and debridement ranges from 8% to 56%, however, depending on whether the infectious organism is Staphylococcus aureus or S. epidermidis (4). Late infections, occurring more 3 to 4 weeks after index surgery, are either latent infections that arose from contamination during initial arthroplasty or hematogenous infections. Late infections that developed as indolent low-grade sepsis are often more difficult to eradicate than those arising more acutely from a hematogenous source. A late infection of a well-fixed TKA with acute onset of symptoms for less than 1 or 2 weeks may sometimes be treated effectively with arthrotomy, polyethylene exchange, thorough synovectomy, and soft tissue debridement with a 6-week course of parenteral antibiotics. This type of treatment is particularly effective with highly sensitive organisms of low virulence in immunocompetent patients (4). However, chronic low-grade infections tend to be more difficult to eradicate by debridement and implant retention. Other options such as serial aspirations, arthroscopic debridement, and chronic antibiotic suppression, alone, are often ineffective and therefore not recommended for treating late infections. We recommend two-stage exchange arthroplasty for chronic or late infections, acute infections by resistant organisms, infection in an immunocompromised or malnourished host, or patients with soft tissue deficiency (5, 6, 7, 8, 9, 10, 11, 12, 13).
Patients in whom an attempt at staged reimplantation has failed are candidates for salvage procedures including resection arthroplasty, knee fusion, and above-the-knee amputation. Patients with extensive medical comorbidities who are unable to tolerate multiple surgical procedures may be best suited to treatment with implant resection and debridement without use of intervening antibiotic spacers. These patients may go on to spontaneous autofusion if treated in a cast. Those with persistent ongoing infection despite multiple attempted staged revision arthroplasties and those with non-healing sinus tract problems, a compromised soft tissue envelope, or extensor mechanism incompetence are also not suitable for reimplantation. If incompetence of the extensor mechanism is observed at the time of implant extraction and debridement, we usually plan for staged fusion.
Whether planning eventual reimplantation, resection arthroplasty, or fusion, an initial meticulous soft tissue and bone debridement, as well as complete removal of polymethylmethacrylate is paramount.
DIAGNOSING DEEP INFECTION
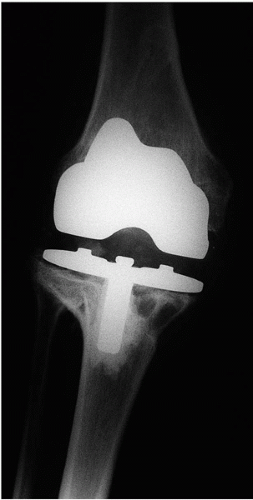
Establishing a diagnosis of deep infection after TKA may be a considerable challenge but is of utmost importance so that appropriate treatment can be initiated (1,14, 15, 16). Purulent drainage, sinus tract formation, fevers, and chills are uncommon in deep knee infection. A methodical application of available diagnostic tests is advisable, but reflexive overutilization of studies is often unnecessary and wasteful of time and resources (14). A careful history regarding the onset of symptoms and a meticulous physical examination will often give important clues as to the source of failure. Radiographs rarely distinguish between septic and aseptic failure, but select serologic studies are very useful for diagnosis (Figs. 22-1 and 22-2). A peripheral white blood cell count is rarely elevated, but a preponderance of polymorphonuclear cells may suggest infection. Used in concert, the sedimentation rate and C-reactive protein (CRP) are more accurate serologic studies, particularly for late infections (14,17, 18, 19). Knee aspiration is the most effective method for identifying a deep knee infection after TKA (17,20, 21, 22). The knee is an easy joint to aspirate and serial aspirations, with the patient off antibiotics for 3 or 4 weeks, may significantly enhance the diagnostic yield. If a patient with chronic insidious symptoms had been put on antibiotics empirically by another physician without having first aspirated and cultured the synovial fluid, then we tend to discontinue antibiotics for 4 weeks (provided symptoms or signs of bacteremia do not develop) and then re-aspirate the knee. Recent studies suggest that a white blood cell count of greater than as little as 2500 to 3120 cells/μL and neutrophil differential >60% to 65% in the knee aspirate carries over 95% accuracy in diagnosing periprosthetic joint infection (17,21,22). Serial aspirations have been shown to increase diagnostic yield of cultured fluid, so in cases of heightened clinical suspicion, serial aspirations are performed (23). Gram stains of the aspirate are often not helpful, but occasionally, bacteria may be seen. We rarely use nuclear studies such as labeled white blood cell scans, although total body technetium scans may be useful in ruling out metachronous infection in a patient with multiple painful prosthetic joints, when one joint implant is infected (24,25). We believe that intraoperative frozen-section histoanalysis can be very useful in identifying occult infection, but it is extremely sensitive
to errors in tissue sampling and depends on the expertise of the histopathologist examining the tissues (26, 27, 28). Thus, institutions with available expertise can benefit from the use of frozen section in select cases. To improve yield and accuracy of frozen-section and intraoperative culture, representative multiple tissue samples from affected regions of the joint must be obtained. Interface tissue between the prosthesis and bone is mostly preferred, but inflamed synovial tissue or granulation tissue can also be useful. Fibrous or fibrin-rich tissue is difficult to interpret and should not be sent. At least two tissue samples should be sent from each knee, and the five most cellular fields should be analyzed under high-power magnification. The presence of more than 10 polymorphonuclear leukocytes per high-power field in five or more fields is almost uniformly consistent with deep periprosthetic infection. Between five and nine polymorphonuclear leukocytes per high-power field may suggest infection but should be considered in concert with other preoperative diagnostic tests.
to errors in tissue sampling and depends on the expertise of the histopathologist examining the tissues (26, 27, 28). Thus, institutions with available expertise can benefit from the use of frozen section in select cases. To improve yield and accuracy of frozen-section and intraoperative culture, representative multiple tissue samples from affected regions of the joint must be obtained. Interface tissue between the prosthesis and bone is mostly preferred, but inflamed synovial tissue or granulation tissue can also be useful. Fibrous or fibrin-rich tissue is difficult to interpret and should not be sent. At least two tissue samples should be sent from each knee, and the five most cellular fields should be analyzed under high-power magnification. The presence of more than 10 polymorphonuclear leukocytes per high-power field in five or more fields is almost uniformly consistent with deep periprosthetic infection. Between five and nine polymorphonuclear leukocytes per high-power field may suggest infection but should be considered in concert with other preoperative diagnostic tests.
Intraoperative cultures, although considered the most reliable determinant of infection, may be negative in 5% to 8% of acute and as many as 20% of chronic or indolent infections (16). When there is a strong clinical suspicion for infection despite negative intraoperative cultures, the knee should be treated as infected. We use a number of important strategies that improve the accuracy of intraoperative cultures. First, to increase the likelihood of organism isolation, preoperative antibiotics are withheld from patients with suspected infection in whom the infecting organism is not identified. (This is different from how antibiotics are given when infection is not suspected, in which case preoperative antibiotics are given within 30 to 60 minutes of tourniquet inflation.) Second, we usually send between four to five tissue samples from various affected regions of the knee. The tissue samples intended for culture are obtained with the use of clean and unused instruments and are transferred into containers without allowing them to come into contact with the gloves or drapes. Once multiple samples from different affected areas are obtained, the culture samples are sent to microbiology labs immediately. The latter strategy is effective in reducing the false-positive rate of infection. The tissues are sent for aerobic and anaerobic culture, which increases the likelihood of identifying not only indolent infection but also polymicrobial infection. Fungal or microbacterial testing is not routinely done, except in unusual cases. After cultures are taken, antibiotics may be administered with deflation of the tourniquet. The choice of antibiotic is based on the results of preoperative aspiration culture. If the infecting organism is not known, a broad-spectrum antibiotic such as vancomycin is administered. Culturing of draining wounds or sinus tracts is not recommended because of the likelihood of false-positive cultures from contamination.
SURGICAL TECHNIQUE
Surgical Exposure
The basic tenets of the surgical approach to the infected knee undergoing arthroplasty are similar to those for all revisions. In the knee with multiple surgical scars, the most lateral incision through which a standard arthrotomy can be performed is most often used. Small skin bridges should be avoided to minimize the risk of soft tissue hypoperfusion and potential for tissue necrosis. Sinus tracts within the margins of the skin incision are elliptically excised down to and through the capsule. Sinus tracts in other areas away from the surgical incision are also excised to minimize the risk of further contamination. An atrophic skin envelope, a multiply scarred knee, or a knee with large sinus tracts should prompt preoperative consultation with a plastic surgeon. In some cases, muscle flap and skin graft coverage may be required during the initial resection arthroplasty. Adequate soft tissue coverage helps promote healing and is critical for eradicating infection.
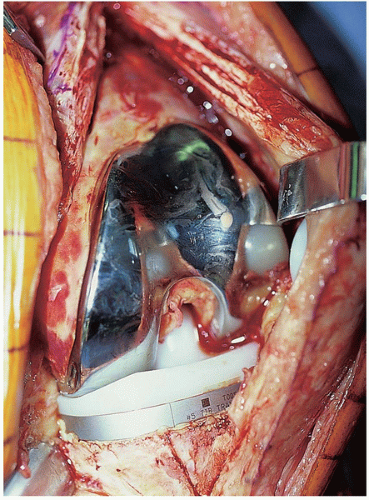
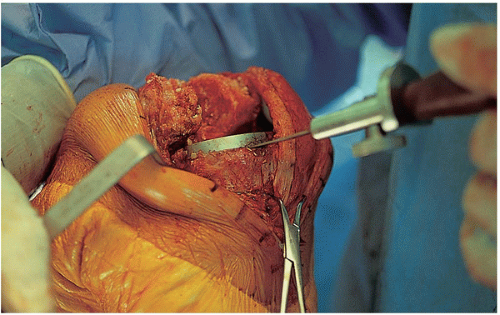
The surgical procedure proceeds in a systematic and organized manner that enables the surgeon to resect all infected tissues. At the time of arthrotomy, cultures are taken both of synovial fluid and inflamed soft tissues (Fig. 22-3). Remnants of all unabsorbed sutures are removed. Thorough synovectomy is performed before flexing the knee. The synovectomy is started from one area of the knee and performed in a stepwise fashion until healthy-appearing tissue is exposed. Lateral capsular tension is applied with the use of two Kocher clamps (Fig. 22-4). The surgeon also débrides the medial gutter and suprapatellar pouch in a similar fashion until healthy and viable tissue remains (Fig. 22-5). Often, extensive synovectomy provides ample exposure to allow flexion of the knee. Occasionally, however, undue tension is placed on the distal insertion of the patellar tendon, and the tibial insert may be removed at the outset to reduce the risk of patellar tendon avulsion (Fig. 22-6). A subperiosteal release of the deep medial collateral ligament, capsular sleeve, and semimembranosus insertion may facilitate anterolateral subluxation of the tibia and also relieve stress on the patellar tendon. The surgeon inserts a towel clamp or 1/8-inch pin through the patellar tendon insertion to further reduce the risk of avulsion (Fig. 22-7). At times, ancillary
extensile approaches are necessary to achieve adequate exposure. Our preference is an inverted V-plasty of the proximal quadriceps tendon. A short oblique incision measuring 1 to 2 cm is directed laterally and distally from the proximal extent of the medial parapatellar incision at an angle of approximately 45 degrees (Fig. 22-8). This approach is safe and generally does not devascularize the proximal edge of the quadriceps tendon, because unlike a formal Coonse-Adams turndown, the superolateral geniculate vessels may be preserved. This approach, coupled with a lateral retinacular release from the inside out, with preservation of the superolateral geniculate vessels, provides ample exposure (Fig. 22-9). Quadriceps advancement is generally not performed. Other proximal releases may be performed, including the Insall snip, although for particularly tight knees, this approach may not be adequate. A tibial tubercle osteotomy, although potentially useful at the time of reimplantation, should be avoided at the time of implant extraction because of the risk of infected nonunion of the tibial tubercle, and the presence of hardware may compromise the ability to completely eradicate the infection.
extensile approaches are necessary to achieve adequate exposure. Our preference is an inverted V-plasty of the proximal quadriceps tendon. A short oblique incision measuring 1 to 2 cm is directed laterally and distally from the proximal extent of the medial parapatellar incision at an angle of approximately 45 degrees (Fig. 22-8). This approach is safe and generally does not devascularize the proximal edge of the quadriceps tendon, because unlike a formal Coonse-Adams turndown, the superolateral geniculate vessels may be preserved. This approach, coupled with a lateral retinacular release from the inside out, with preservation of the superolateral geniculate vessels, provides ample exposure (Fig. 22-9). Quadriceps advancement is generally not performed. Other proximal releases may be performed, including the Insall snip, although for particularly tight knees, this approach may not be adequate. A tibial tubercle osteotomy, although potentially useful at the time of reimplantation, should be avoided at the time of implant extraction because of the risk of infected nonunion of the tibial tubercle, and the presence of hardware may compromise the ability to completely eradicate the infection.
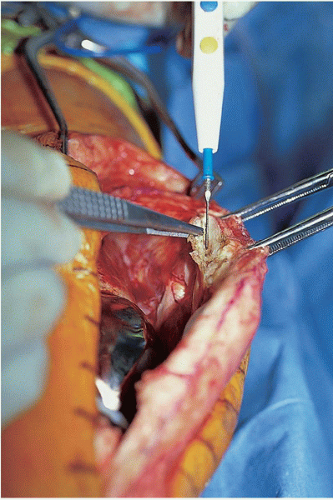 FIGURE 22-4 Synovectomy of the lateral soft tissues and gutter can be performed with the knee in extension. |
 FIGURE 22-5 A rongeur may be used to débride the inflamed periprosthetic tissues, and a medial synovectomy may be performed with the knee extended. |
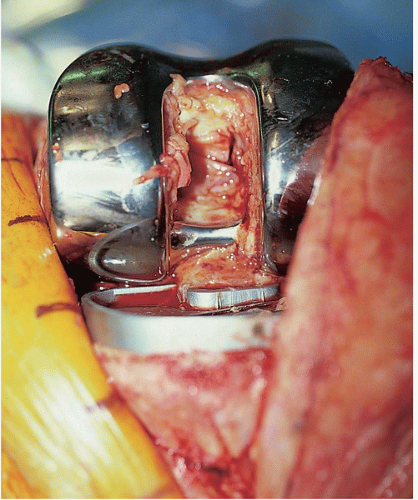 FIGURE 22-6 The polyethylene insert may be removed to facilitate flexion of the knee and exposure of the posterior aspect of the joint. |
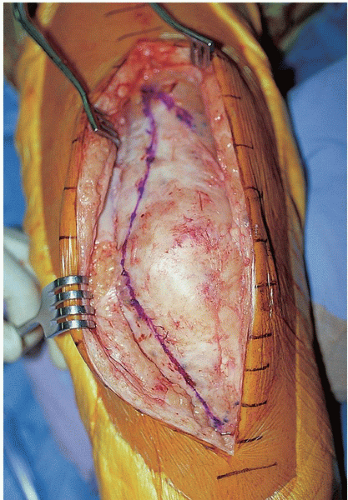 FIGURE 22-8 The planned arthrotomy incision is marked and includes a small lateral limb that may be used if an extensive approach is necessary for exposure. |
IMPLANT REMOVAL
Once adequate exposure is obtained, removal of components begins. Removing well-fixed implants may be arduous, but uninfected bone stock and collateral ligament attachments need to be preserved with care. Attempts at component extraction are withheld until the interfaces are meticulously cleared and freed from underlying bone to minimize the risk of inadvertent bone removal. The interface edges beneath the components are demarcated with either a rongeur or small curette. We prefer to remove the femoral component first, as this often facilitates exposure of the tibia and avoids deposition of cement fragments and other debris into the medullary canal of the tibia. The femoral component can be removed with a combination of flexible and rigid osteotomes or a Gigli saw (Figs. 22-10 and 22-11). If applicable, all cement and necrotic tissue must be removed from the exposed bone surfaces and lug holes (Fig. 22-12). The tibial and patellar components are removed with a combination of small saw blades and osteotomes (Figs. 22-7 and 22-13). All of the cement is removed with a saw, osteotome, and burr (Fig. 22-14). When all implants are removed, synovectomy of the posterior aspect of the joint is performed.
Debridement of necrotic or infected bone is critical. Although preservation of ligamentous attachments and bone stock is important, thorough debridement of infected or devitalized tissue takes precedence to clear the infection. It is plausible that a high percentage of recurrent infections are caused by inadequate debridement of infected bone. Suspicious bone should be cultured and histologically analyzed to rule out osteomyelitis. Occasionally, further surgery is necessary to more thoroughly address foci of osteomyelitis.
After removing all cement and necrotic tissue, the knee is irrigated copiously with 6 to 9 liters of pulsatile lavage using solution that contains bacitracin and/or polymixin (Fig. 22-15). Before implanting the antibiotic spacers, the extent of bone loss and the integrity of the collateral ligaments should be assessed in an effort to prepare preliminarily for eventual reimplantation.
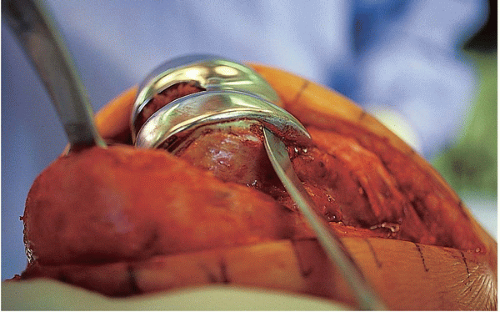 FIGURE 22-10 After the prosthetic interfaces are cleared and the interface edges demarcated, the femoral component is removed with an osteotome. |
 FIGURE 22-12 After femoral prosthesis extraction, cement and fibrous tissue are removed from the distal femur. Additionally, necrotic bone and inflamed tissue should be removed at this stage. |
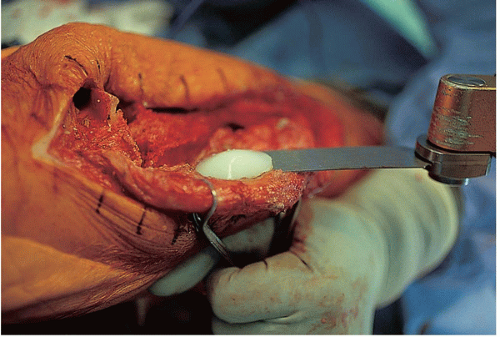 FIGURE 22-13 The patella is removed with an oscillating saw, and all underlying cement is removed from the lug holes using a high-speed burr, osteotome, or saw. |
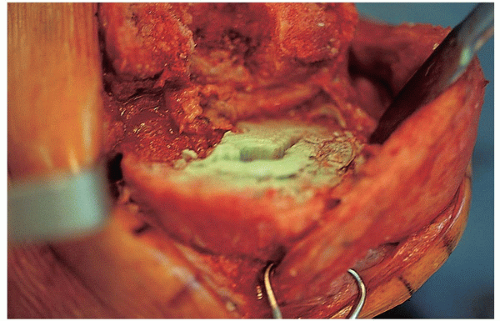 FIGURE 22-14 After prosthesis removal, tibial bone stock should be assessed and any further cement removed. |
INTERVAL ANTIBIOTIC SPACERS
We prefer two-stage revision with an interval period of antibiotic-impregnated cement spacers for most late infections (5




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree