Split Posterior Tibial Tendon Transfer
David A. Spiegel
James J. McCarthy
DEFINITION
The equinovarus deformity involves hindfoot equinus and varus and results from imbalance between inversion (tibialis posterior, tibialis anterior, or both) and eversion of the foot.
The deformity may interfere with ambulation, orthotic wear, or both.
Split tendon transfers are used in patients with spastic muscle imbalance to prevent overcorrection or production of the opposite deformity, usually in children with cerebral palsy who have spastic hemiplegia.1 The procedure weakens a deforming force while augmenting a weakened muscle. In contrast, patients with a flaccid equinovarus deformity, due to poliomyelitis or other causes, are typically treated by transfer of an entire muscle(s) to augment the strength of selected muscle groups.
ANATOMY
The tibialis posterior muscle originates from the posterolateral aspect of the tibia, the interosseous membrane, and the medial fibula.
Although the main insertion is into the tuberosity of the navicular, fibers also insert onto the cuneiforms, the second through fourth metatarsals, the cuboid, and the sustentaculum tali.
The gastrocnemius muscle originates from the posterior surface of the distal femur, and its tendon blends with the tendon of the soleus muscle to form the Achilles tendon, which then inserts on the posterior tuberosity of the calcaneus.
The soleus muscle takes origin from the posterior portion of the upper third of the fibula, the fibrous arch between the tibia and the fibula, and the posterior aspect of the tibia. The broad tendinous portion along the posterior aspect of the soleus joins with the gastrocnemius tendon to form the Achilles tendon.
PATHOGENESIS
The deformity results from muscle imbalance between plantarflexion-inversion (strong) and dorsiflexion-eversion (weak). Spasticity of the tibialis posterior, the tibialis anterior, or both may be responsible for the imbalance.
NATURAL HISTORY
The deformity is initially dynamic, with a full range of motion on physical examination.
A myostatic contracture often develops over time, evidenced by the inability to achieve a full passive range of motion.
Tethering of growth may subsequently result in structural bony deformities such as hindfoot varus.
The equinovarus deformity may result in pathologic changes in both the stance and swing phases of gait, including impaired clearance during swing phase, inability to preposition the foot in terminal swing, and loss of stability during stance phase.
PATIENT HISTORY AND PHYSICAL FINDINGS
Patients present with progressive gait disturbance, with or without pain, and may have difficulty wearing orthotics.
Pain is due to the abnormal stress distribution on the plantar surface of the foot and is commonly experienced over the distal fifth metatarsal and the lateral border of the foot. Calluses may be observed laterally.
Recurrent ankle sprains may occur as the hindfoot rolls into varus. This may also be associated with a tarsal coalition.
In addition to a comprehensive neurologic examination, examination of the spine and both extremities, the physical examination focuses on observational gait analysis, the presence of and degree of spasticity in the individual muscle groups, the range of motion (active and passive) of the foot and ankle, and the selectivity of motor control.
Observational gait analysis focuses on the alignment of the foot and ankle during both the swing and stance phases of gait.
During swing phase, the foot is inverted and plantarflexed, which impairs clearance.
The inability to maintain the foot in neutral plantarflexion-dorsiflexion during midswing may be due to muscle weakness (tibialis anterior), muscle spasticity (tibialis anterior or posterior, gastrocsoleus), or a fixed equinovarus deformity.
There is inadequate prepositioning of the foot for weight acceptance during terminal swing.
Initial contact often occurs over the lateral forefoot (no heel contact), or over the lateral border of the foot, and the foot rolls into varus, which interferes with stability during stance phase.
The equinovarus deformity may also contribute to intoeing (internal foot progression angle).
The presence and degree of spasticity should be documented.
The most common system for grading is the modified Ashworth scale. Each muscle is tested by gentle stretch; for example, spasticity of the tibialis posterior is assessed by everting the foot, whereas the gastrocsoleus complex is assessed by dorsiflexion.
The strength of individual muscle groups should be graded if possible.
Testing the passive range of motion determines whether the deformity is dynamic (full passive range of motion) or whether there is a myostatic component (restriction of passive range of motion).
Although bench examination provides a useful estimate of motion, an examination under anesthesia provides the
most accurate evaluation, as spasticity is eliminated. Such an examination is always performed at the time of surgery to finalize the treatment plan, as a full passive range of motion is a prerequisite for a tendon transfer.
For patients with an equinovarus deformity, the examination focuses on the degree of passive eversion and dorsiflexion. Equinus contracture is often limited to the gastrocnemius muscle but may also involve the soleus muscle.
The Silfverskiöld test evaluates the contribution of each component of the gastrocsoleus complex to an equinus contracture, and the amount (in degrees) of passive dorsiflexion is quantified with the knee both flexed and extended. The degree of passive dorsiflexion with the knee extended indicates the absolute magnitude of contracture from the gastrocnemius and soleus. Flexion of the knee relaxes the gastrocnemius muscle and allows the contribution of the soleus to be quantified.
Selectivity of motor control is commonly impaired in children with cerebral palsy and is tested by asking the patient to contract an isolated muscle group against resistance. This is graded as normal if the patient can isolate the individual muscle and no “overflow” movement is observed in other muscle groups of the same limb. Most commonly, movements of more than one muscle group, or the entire limb, are elicited when testing individual muscle groups.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Although imaging studies are not routinely obtained, plain radiographs of the foot may be helpful in the presence of a fixed deformity.
Weight-bearing anteroposterior (AP) and lateral views are reviewed, and a Harris heel view may be considered to evaluate the degree of hindfoot varus in the weight-bearing position.
Instrumented motion analysis (gait analysis) is used in many centers to assist with surgical decision making.
Slow-motion video is an important component of the assessment and supplements the findings on observational gait analysis.
Dynamic electromyelography (EMG) monitors the electrical activity of the tibialis posterior and tibialis anterior throughout the gait cycle, determining whether individual muscles act out of phase or whether they are continuously active throughout the gait cycle.13 Although a surface electrode may be used to assess the tibialis anterior, monitoring of the tibialis posterior requires insertion of a fine needle electrode.
One study determined that the deformity was due to the tibialis posterior in 33%, the tibialis anterior in 34%, or both (31%).9
Findings on pedobarography include increased pressure across the lateral midfoot, decreased pressure on the heel at the time of initial contact, and increased pressure on the lateral border of the foot throughout stance phase.
NONOPERATIVE MANAGEMENT
Specific aspects within a comprehensive physical therapy program include stretching exercises to maintain or improve range of motion and strengthening exercises to reduce dynamic muscle imbalance.
An ankle-foot orthosis is often required to maintain alignment of the ankle and hindfoot during ambulation.
The orthotic facilitates clearance during swing phase by maintaining the foot in a neutral position, prepositions the foot for initial contact with the ground, and promotes stability during stance phase.
Night splinting may help to prevent myostatic contracture.
Injection of botulinum toxin A (Botox or Dysport) into the tibialis posterior, the gastrocsoleus, or both results in a reversible chemical denervation that decreases spasticity for about 3 to 6 months.
In addition to reducing dynamic muscle imbalance, a temporary reduction in spasticity may facilitate stretching exercises, improve bracing tolerance, and delay the need for surgical intervention.
SURGICAL MANAGEMENT
Surgical treatment of the spastic equinovarus foot is offered when the deformity impairs ambulation, interferes with bracing, or both.
The goal of tendon transfer is to balance the muscle forces across the hindfoot to maintain a neutral position during the swing and stance phases of gait. A split tendon transfer is preferred as transfer of the entire tendon is associated with a significant risk of overcorrection.
A normal passive range of motion is a prerequisite. In the presence of fixed soft tissue or bony deformity, concomitant muscle lengthening, with or without osteotomy, may be required to restore motion and alignment.
Although an instrumented motion analysis with dynamic EMG will enable the treating surgeon to identify whether the tibialis posterior, the tibialis anterior, or both is/are contributing to the deformity, this technology is not always available. The clinical indications suggested for split tibialis posterior tendon surgery include hindfoot varus during both the stance and swing phases of gait. In contrast, overactivity of the tibialis anterior typically produces varus/supination of the midfoot/forefoot during swing phase.
It has been suggested that the procedure be delayed until at least 4 to 6 years of age, and one recent report suggested that consideration should be given to delaying split tendon transfer beyond the age of 8 years if possible as there may be a greater risk of recurrence.2
Lengthening of the tibialis posterior muscle may be considered in milder deformities, especially in young patients. Techniques include a distal Z-lengthening or a proximal intramuscular recession. Recognize that it may be very difficult to perform a split tendon transfer if a Z-lengthening has been performed previously.
Several techniques have been described for split tibialis posterior transfer.
The most common involves transferring the split tendon (posterior to the tibia and fibula) to the peroneus brevis, either at its insertion or just behind the lateral malleolus. This approach focuses on balancing inversion-eversion but does not address dorsiflexion weakness (FIG 1).
An alternate technique, which may be considered when there is inadequate active dorsiflexion, involves anterior transfer of the split tendon through the interosseous membrane to the peroneus brevis (FIG 2A,B) or the lateral cuneiform (FIG 2C).
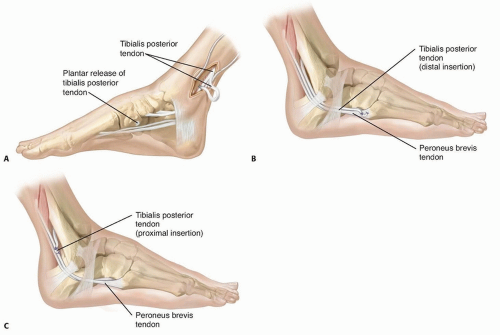
FIG 1 • In the technique described by Kaufer,5 the split tendon is routed behind the tibia and fibula (A) and inserted into the peroneus brevis tendon (B). C. Alternatively, the split tendon can be woven into the peroneus brevis just behind the lateral malleolus. This approach is easier and works as well when the tendon is not long enough.
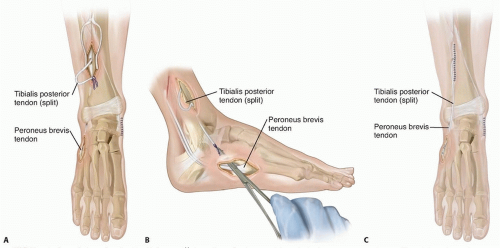
FIG 2 • In the technique described by Mulier et al,11 the split tendon is passed through the interosseous membrane (A) and through a subcutaneous tunnel to insert into the peroneus brevis tendon (B). C. Saji et al16 transferred the split tendon through the interosseous membrane into the lateral cuneiform.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access






