CHAPTER 109 Spinal Ablative Techniques for the Treatment of Chronic Pain Conditions
INTRODUCTION
The interruption of pathways of the nervous system concerned with pain is a classical approach to the relief of intractable pain disorders, which stems from neurosurgeons having been trained with a primary understanding of anatomy and, to a lesser extent, an appreciation of neurophysiology. While successful when implemented in the correct clinical setting, there have also been significant complications with these procedures, including return of pain and sometimes worsening of pain, and/or the evolution of neuropathic pain syndromes in many patients treated with these procedures.
Historical aspects of pain management
The origin of the word ‘pain’ is the Latin word poena, meaning punishment. The early concept of pain as a form of punishment for sinful activities is as old as humankind.1 Early healers used a wide range of modalities to attempt the control of pain, including herbal medicines and the application of electric fish.2 Decartes’ description of pain conduction from peripheral damage through nerves to the brain led to the first scientific understanding of pain.3 The first plausible surgical management of pain was on an anatomic basis where nerves were cut in attempts to denervate the painful areas of the body. These methods were a direct byproduct of the understanding of pain transmission during the scientific revolution. It was not until the Wall and Melzack Gate Control Theory that a sound scientific basis for pain mechanism was formulated.4 Shortly thereafter, initial efforts were made to modulate pain at peripheral nerve, spinal cord,5,6 and other targets such as brain stem7 and, more recently, the cerebral cortex.8
The diagnosis of pain
Long before the different mechanisms of chronic pain were defined, neurosurgeons distinguished between chronic pain due to cancer and chronic pain of ‘benign’ origin. Currently, a more helpful way to differentiate chronic pain is through physiology: namely, nociceptive and neuropathic pain syndromes. The difference between these two categories is the absence of a continuous nociceptive input through pain receptors in neuropathic pain syndromes. It is key to recognize that a given patient may have features of more than one pain physiology. Weir Mitchell first described and named causalgia as a regional pain disorder associated with both motor and sensory disturbance.9 Since then, many and often confusing terms have been used to describe chronic neuropathic pain syndromes. In 1994, the International Association for the Study of Pain (IASP) adopted the term complex regional pain syndrome (CRPS) to replace the terms reflex sympathetic dystrophy (RSD) and causalgia.10 Complex regional pain syndrome is further divided into type 1 and type 2, representing RSD and causaglia, respectively. CRPS may exist in a state of flux and, regardless of whether the patient suffers from CRPS 1 or 2, there may be sympathetically maintained and independent features involved. While many theories exist, the definitive pathophysiology and etiology of CPRS remains unclear.
A correct physiologic diagnosis can only be obtained through detailed history and physical examination. It may be necessary to obtain diagnostic studies such as computed tomography (CT) scan, margnetic resonance imaging (MRI), and electromyogram/nerve conduction velocity (EMG/NCV). Diagnostic spinal procedures such as selective root blocks, sympathetic blocks, and discography will often further aid the clinician. There is no confirmatory test or procedure for CRPS 1, and this diagnosis can only be attained through a clinical examination. An early and specific refinement of the pain diagnosis is necessary and valuable, as it directs care of the patient.
SPINAL RHIZOTOMIES AND GANGLIONECTOMIES
Historical background
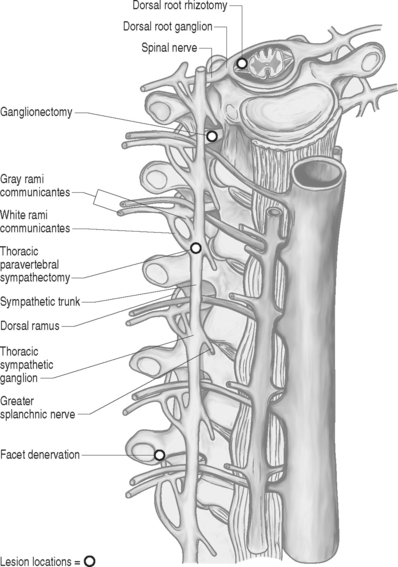
Dorsal root rhizotomy was first attempted for the relief of intractable pain by Abbe in 1889.11 The operation was based on the concept that afferent signals are conveyed via the dorsal roots while efferent signals are conveyed via the ventral roots.12,13 There now exists a large body of evidence supporting the existence of as much as 30% afferent axons passing through the ventral roots.14–16 Dorsal rhizotomies or ganglionectomies are primarily indicated for treatment of pain syndromes involving the neck, trunk, abdomen, and perineal region. Persistent post-thoracotomy or postlaparotomy pain is a frequent indication for these procedures, as well as treating malignant pain syndromes from pleural based or apical lung lesions. Several variations of spinal root surgery have been developed over the century, though the methods have experienced a long decline due to the disappointing outcomes of most series.17,18 The procedure is contraindicated for treating extremity pain in a functional limb, as complete or near-complete functional paralysis will occur because of absence of sensory input. Extensive sectioning of sacral posterior root also needs to be performed with selectivity as interference with sphincter and sexual function can occur.
Pertinent anatomy
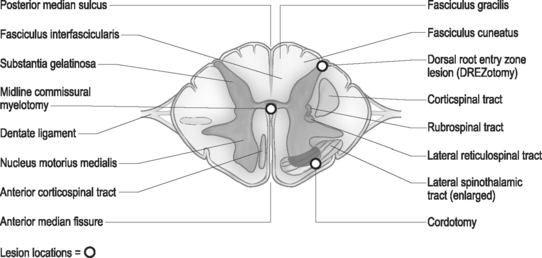
The spinal root anatomy begins with the formation of the anterior roots, which are formed by three to five rootlets emerging from the anterolateral sulcus; the posterior roots are formed by three to ten rootlets which penetrate into the dorsolateral sulcus (Fig. 109.1). The dorsal and ventral roots are separated by the dentate ligaments, though they are grouped together prior to leaving the thecal sac. Microscopic anastomotic branches exist that pass from one rootlet to another. As the roots approach the intervertebral foramen, both the ventral and dorsal roots are situated in a common dural sleeve. In the intervertebral foramen the dorsal ganglion can be identified. At this point the subarachnoid space is sealed by the arachnoid trabeculae and no cerebrospinal fluid (CSF) is contained.19 Distal to the dorsal ganglion and lateral to the foramen, the spinal nerve is formed which will then bifurcate into ventral and dorsal branches.
Procedure
Prior to the procedure, a selective nerve root block may aid in determining if rhizotomy or ganglionectomy may provide any benefit. The operation is performed under general anesthesia with the patient in the prone position and may be performed via an intradural (dorsal rhizotomy) or extradural (ganglionectomy) approach. The desired spinal segments are exposed. In the case of dorsal rhizotomies, laminectomies are performed and a midline dural opening is made. The dorsal rootlets of the desired segments are then sectioned sharply. A technique described by Sindou in 1972 aimed to interrupt selectively small-diameter nociceptive fibers at their radicular entrance into the spinal cord.20 This technique of selective dorsal root rhizotomy allows for preservation of lemniscal fibers that may avoid secondary appearance of pain and leave intact substrates that can be utilized for neuromodulatory procedures.21 For ganglionectomies, foraminotomies are performed to expose the desired dorsal root ganglia. The ganglia are then carefully dissected free from the surrounding tissue and the dorsal root is divided proximal to the ganglion. Following this, the ganglion is gently elevated and the distal connection to the root is sectioned. In most cases, the ventral root can be spared. Important aspects from a purely technical point of view include verification of the level of lamienctomy, root identification by anatomical, radiographic, and physiological means, selection of roots by stimulation, recognition of anastomoses between roots, and sparing of radicular arteries. Other technical variants exist such as extradural spinal root ganglion resection as described by Scoville in 1966, which avoid the opening of the dura.
An alternative to the open surgical technique’s proximal radiofrequency thermocoagulation of primary spinal trunk and ganglion has been described; Uematse et al. proposed a percutaneous technique for lesioning the dorsal root ganglion and rootlets.22
Outcomes
The variability of clinical results of posterior rhizotomy and ganglionectomy warrants the cautious use of these denervation interventions and should only be used after nondestructive techniques have been exhausted. For lumbar radiculopathies, there is about a 30% success rate at 1 year.23 The results for postherpetic neuralgia are no better, with success rates at less than 30%.24 And the results for non-specific relief of chronic pain are extremely poor in the long term.25 A review of the literature reveals that results are generally less than 50% for good pain reduction with limited long-term follow-up. Unfortunately, these procedures produce a complete denervation of one or more spinal segments, thus precluding the patient from potential future neuromodulation. Because of this, and the general availability of long-acting opiate analgesics, these procedures are not generally recommended.
DORSAL ROOT ENTRY ZONE LESIONS (DREZOTOMY)
Historical background
During the early 1960s, research into pain focused attention on the dorsal root entry zone (DREZ) as the first level of modulation for the cessation of pain.26 What is known of these pathological mechanisms is that the cells in the dorsal root ganglion become hyperactive and send nociceptive impulses via the spinothalamic pathways. Based on this understanding, Sindou performed the first DREZ operation in 1972 for pain caused by Pancoast-Tobias syndrome.27 Others such as Nashold and Ostdahl placed thermal lesions into the substantia gelatinosa of the spinal cord for the treatment of nonmalignant pain.28 In view of the complex anatomy and lack of histological confirmation, the target was referred to as the DREZ. This region in the spinal cord is currently recognized as a sophisticated structure for the modulation of pain and continues to be utilized for ablative techniques, either open or percutaneous.
Pertinent anatomy
Using cytoarchitectural criteria, in 1952 Rexed divided the gray matter of the spinal cord of the cat into ten separate cell layers. Similar laminar patterns were confirmed by Schoenen.29 The uppermost lamina (I–V) are pertinent to the DREZ procedure. The major nociceptive input is distributed to layers I, II, and V (layers I and II representing the substantia gelatinosa), with their second-order neurons giving rise to the spinothalamic tract. Neurons in layers III and IV receive non-noxious inputs from the periphery and project to the dorsal column nuclei. Situated dorsolateral to the dorsal horn is the tract of Lissauer, an intersegmental longitudinal spinal tract with multiple collaterals to Rexed layers I and II. This tract plays an important role in the intersegmental modulation of the nociceptive afferents. Its medial part transmits the excitatory effects of each dorsal root to the adjacent segments and its lateral part conveys inhibitory influences to the substantia gelatinosa.30 The DREZ procedure is directed at destroying the Rexed layers I, II, and V and the medial portion of the tract of Lissauer which coordinates sensory information.
Procedure
The surgical technique of thermal coagulation for DREZ lesions has been described in detail by Nashold and collaborators and extensively published.31 With the patient placed under general anesthesia and in the prone position, laminectomies or hemilaminectomies are performed over the involved regions of the spinal cord. In the cervical region, the localization of the appropriate level is one level rostal to the dermatomal localization, and in the thoracic region two to three veterbral segments rostal to the affected dermatomes (Fig. 109.2). Lumbar and sacral segments are localized through laminectomies at T10 through L1 for exposure of the conus medullaris. The operating microscope is utilized throughout the procedure following the dural opening. Each dorsal root is composed of several small rootlets which enter the cord at the postointermediolateral sulcus at the margin of the dorsal columns. Identification of the root and corresponding cord level can be confirmed with electrophysiologic monitoring. Following the identification of the rootlets of each root, though this may be difficult at the level of the conus, the lesions are made in the dorsal root entry zone. The lesions are created over an additional one to two segments above and below the affected roots to ensure adequate coverage of the painful segments. Bilateral lesioning should be avoided in patients with good neurologic function below the lesion, as deterioration in proprioception, motor, or bladder and bowel function can occur.
Outcomes
The DREZ procedure has been implemented for a large series of deafferentation pain syndromes. Results from several series report a 54–79% success rate for the procedure.32–34 Probably the single best indication for the DREZ lesioning is pain following brachial plexus avulsion. Long-term pain relief for this procedure for brachial plexus avulsion pain reaches 70%.35 The major complication with thoracic spinal DREZ operation is weakness in the ipsilateral leg due to injury of the corticospinal tract; this is seen in 5–10% of patients,36–38 though this may occur with other sites of DREZotomy.
While the DREZ operation has been used in an attempt to treat a multitude of pain conditions, its current indications are very specific. It is best used to treat deafferentation pain (as seen with brachial plexus avulsion), limited cancer pain (as seen with a Pancoast tumor), segmental pain after spinal cord injury, peripheral nerve pain (seen with nerve injuries and phantom limb or stump pain), and postherpetic pain.39 These authors do not utilize this procedure, however, for other than segmental pain following spinal cord injury because neuromodulation is commonly usable in most of the other painful phenomenon listed and neuromodulation has a superior safety profile.
OPEN ANTEROLATERAL CORDOTOMY
Historical background
In 1889, Edinger40 first described the anatomy of the spinothalamic tracts, though functional correlation was not discovered until Spiller reported his findings in 1905.41 The first anterolateral tractotomy was performed by Martin at the suggestion of Spiller in 1911 for management of pain in man.42 Eventually, both in Europe and America, the surgical cordotomy became a standard neurosurgical procedure for the treatment of pain. Mullan43 developed the technique for percutaneous cordotomy using a radioactive strontium needle in 1963 where the lesion can be made without the necessity of general anesthesia. Rosomoff44 further refined the technique using a radiofrequency needle electrode system. Additional refinements to the procedure have included myelography45 to outline landmarks, CT guidance,46 and electrical impedance monitoring.47 While the operation has remained in essence the same as that introduced by Spiller and Martin, much effort over the decades has continued to be devoted to lesioning the anterolateral quadrant of the spinal cord. Both open and percutaneous techniques will be discussed.
Pertinent anatomy
Neuroanatomical and physiological aspects of nociceptive pathways have been extensively studied in animals and humans. Within close proximity to the spinothalamic tract lie many other ascending and descending tracts, damage to which leads to many of the complications of cordotomy. The corticospinal tract is located posteriorly and injury to it results in ipsilateral weakness. Overlying the spinothalamic tract is the ventral spinocerebellar tract and injury of that pathway may produce an ipsilateral ataxia of the arm. Knowledge of the location of descending respiratory pathways is not just theoretical, as one of the most feared complications of a high bilateral cordotomy is respiratory dysfunction. Typically, the patient is capable of voluntary but not involuntary respiration, and consequently dies during sleep because of destruction of the system for automatic respiration (Ondine’s Curse).48
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree