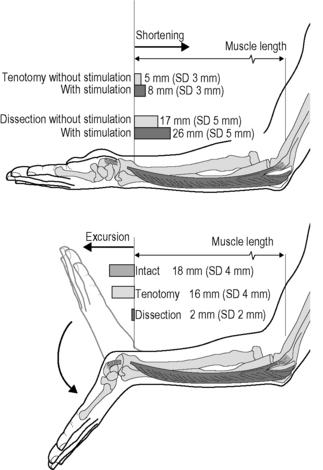
5.4 Spastic paresis Spastic paresis is a common term to denominate typical presentations of the motor disorders in a specific type of cerebral palsy. By definition, cerebral palsy (CP) describes a group of disorders of development of movement and posture, causing activity limitation, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain (Bax et al. 2005). In contrast to most other clinical disorders described in this book, CP is a neurological disorder that does not directly affect fascia or other connective tissues. From this perspective, this chapter deals with fascia-related phenomena in a clinical disorder that does not primarily affect fascia itself. Spasticity is a neurological symptom characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks resulting from hyperexcitability of the stretch reflex (Smeulders & Kreulen 2007). Although the term spasticity is commonly added to the diagnosis of CP, it is seldom the vanguard of its clinical presentation. In the classical perspective, the muscles susceptible to spasticity are supposed to be insufficiently opposed by paretic antagonists and yield a preferred posture of the extremity towards endorotation, flexion, pronation and adduction. During active use of the extremity, interplay between agonistic and antagonistic muscle groups yields a new equilibrium resulting in a limited available range of motion, typical disturbed movement patterns and, eventually, awkward postures based on this equilibrium, where spasticity might be difficult to provoke as a clinical symptom. As such, these joint positions are not necessarily static contractures in the classical sense. For a sizable part, they can acutely be altered in rest, after careful manipulation or under anesthesia. The role of fascia in this mechanism is underexposed in literature. How are the functional properties of intra-, inter- and extramuscular fascial connective tissues involved in this altered musculoskeletal balance? Many of the efforts to provide evidence have been directed at alleged structural changes of spastic muscles as an adaptive response to pathological conditions. Hence, spastic muscles have been considered to be atrophied as a consequence of disuse (Gracies 2005), hypertrophic and fibrotic as an adaptive response to hypertonicity, increasing the amount of collagen within the muscles (Booth et al. 2001), while the long-standing shortened position of the joints has been proposed to cause structural shortening of both intramuscular connective tissues, muscle fibers, and joint capsules (Tardieu et al. 1982; Botte et al. 1988; Fry et al. 2007; Pontén et al. 2007). The role of fascia in spastic paresis has, as such, long been acknowledged. However, controversy exists, as most histological studies fail to prove that spastic muscles are fibrotic and structurally shortened. A few reports even showed biopsies of spastic muscle containing normal amounts of connective tissue (Romanini et al. 1989; Ito et al. 1996; Marbini et al. 2002). Partly in contrast, others reported an increased amount of connective tissue in some of the biopsies from several spastic muscles. However, even in those studies 50% of muscle biopsies were considered normal or only showed a limited abnormality, despite “the presence of static and dynamic contractures” involving the target muscles. Likewise, among muscle biopsies that did indicate fibrosis were those of patients who clinically did not show contractures (Castle et al. 1979; Rose et al. 1994). On the other hand, a sole report indicates a significant correlation found between clinically measured muscle tone and the amount of collagen in spastic muscle biopsies (Booth et al. 2001). Likewise, it has been more and more accepted that loss of serial sarcomeres within muscle fibers as a cause for structural shortening of muscle is not a common finding in spastic muscles, with muscle atrophy present in some, but not all (Fry et al. 2007; Pontén et al. 2007). Mechanical testing of spastic muscle tissue (Lieber et al. 2003) and direct measurement of passive length-force characteristics of partially dissected human spastic flexor carpi ulnaris muscle (FCU) (Smeulders et al. 2004), did not show unusual muscular characteristics that would explain the abnormal wrist position. Therefore, this implies that adaptation of muscular characteristics per se may not cause the joint limitation. Interestingly, we noticed that tenotomy of FCU did not result in a major retraction of the muscle to slack length (even if the muscle was activated maximally). Instead, on tenotomy and after activation FCU shortened a little, but remained relatively close to its former insertion (Fig. 5.4.1) (Kreulen et al. 2003). Even more surprisingly, as the wrist was moved passively from flexion to extension during surgery, tenotomized FCU (not spanning the wrist any longer) was lengthened almost to a similar degree as the intact FCU, showing that the FCU is connected to structures that span the wrist via epimuscular or epitendinous connective tissues (creating a distally directed myofascial load on FCU; for further explanation see Chapter 3.2). Even after tenotomy, such connections pull the FCU along on passive wrist extension. On wrist flexion, FCU retracted elastically so the process was repeatable during cyclic movement of the wrist. Apparently, the connective tissues involved were so stiff that they could transmit the full FCU force, and strong enough so that they did not break on such force exertion. Such FCU length changes after tenotomy diminished greatly, but did not fully disappear after partial dissection in distal-proximal direction (along 50–60% of the muscle belly, necessary for subsequent tendon rerouting). These results place the concept regarding the role of the fascia in spastic joint positions in a whole new perspective.
Introduction
Spastic muscles
Observations during surgery
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine