Soft Tissue Coverage of the Pelvis and Sacrum: Hemipelvectomy and Pedicled Flap Coverage
Alex Senchenkov
Robert Esther
Franklin H. Sim
Steven L. Moran
Extensive defects of the pelvis and sacrum can result from tumors, ablation, and severe trauma. Due to limited amounts and relative immobility of the pelvic soft tissue, these defects may pose a serious reconstructive challenge. Until the late 1970s, the majority of large pelvic tumors were treated with external hemipelvectomy. Advances in imaging, chemotherapy, and radiation therapy, as well as improvements in resection and reconstructive techniques, have greatly reduced the need for radical lower extremity amputations, allowing limb preservation in a majority of cases.
Historically, buttock tumors were not amenable to a classic hemipelvectomy and just a few decades ago were considered unresectable. Likewise, extensive buttock defects inflicted by trauma, infection, or end-stage pressure ulcers in paraplegics could not be effectively reconstructed. Secondary intention healing frequently resulted in protracted hospital course, extensive scarring, contractures, and unstable soft tissue coverage. Many of these patients were bound to years of ongoing wound care and immobility.
External hemipelvectomy denotes removal of the hemipelvis with affected lower extremity by disarticulation of the pubic symphysis and the sacroiliac joint. Because external hemipelvectomy resulted in major functional impairment, limb-sparing procedures removing part or all innominate bone with preservation of the extremity have been advocated. These pelvic resections are referred to as internal hemipelvectomies.
Large, composite pelvic defects associated with internal hemipelvectomies are more challenging to reconstruct than the soft tissue defect typically created in external hemipelvectomy patients for two main reasons. First, following removal of bony hemipelvis in external hemipelvectomy, a large amount of soft tissue of the buttock or proximal thigh becomes available for reconstruction. Second, a decrease of the pelvic volume obliterates the dead space.
Sacral resections are performed as a part of extended external hemipelvectomy for musculoskeletal sarcomas and, as such, reconstructed as a part of hemipelvectomy closure. Isolated sacral defects result from composite pelvic resections for locally advanced anal and rectal malignancies or tumors intrinsic to the sacrum such as sacral chordomas and sarcomas.
Indications/Contraindications
When embarking on treatment of pelvic sarcomas, three important questions should be borne in mind.
Is this patient operable, i.e., can the individual medically withstand a major oncologic resection?
Is this tumor resectable, i.e., can this patient be rendered disease-free surgically?
Can the residual defect or deformity from the proposed resection be reconstructed in a functionally satisfactorily manner with stable soft tissue coverage?
The answers to these questions have to be determined during preoperative evaluation by the surgical oncologist, reconstructive surgeon, and anesthesiologist. Resection of the tumor with negative margins is the only reliable means of obtaining a cure in cases if tumor.
Internal hemipelvectomy is indicated in cases of localized tumor where margin negative resection of the tumor is possible with preservation of the lower extremity. If clean margins cannot be achieved, external hemipelvectomy should be performed. Main indications for external hemipelvectomy are: large tumors involving multiple compartments unresponsive to neoadjuvant therapies, contamination of compartments from pathologic fracture, or failed previous resection, a nonviable extremity. Nononcologic external hemipelvectomy may be performed in the cases of uncontrolled pelvic osteomyelitis, traumatic hemorrhage, and failed aorto-femoral revascularizations (Table 35-1).
Wound complication rates following hemipelvectomy are notoriously high and have been reported to range from 20% to 80%. Proper technical execution of the procedure and the use of well-designed skin and muscle flaps can minimize postoperative wound morbidity.
Although infrequently, pelvic and sacral resections are performed en bloc with pelvic visceral structures for locally advanced rectal and gynecologic malignancies eroding or invading the skeletal pelvis. When such pelvic resection involves removal of a part of the pelvis or sacrum, it is referred to as composite resection. Any type of external hemipelvectomy performed in continuity with
visceral structures is known as compound hemipelvectomy. Due to the aggressive nature of these tumors, the disease has to be limited to the pelvis and extensive imaging is required to select the patients that can benefit from these extensive operations.
visceral structures is known as compound hemipelvectomy. Due to the aggressive nature of these tumors, the disease has to be limited to the pelvis and extensive imaging is required to select the patients that can benefit from these extensive operations.
Table 35-1. Basic Tumor Flap Principles | |
|---|---|
|
Primary sacral tumors such as chordomas and sarcomas are relatively uncommon. The majority of these tumors are low-grade malignancies. They infrequently metastasize and therefore local control becomes important. Reconstruction of these defects with flaps facilitates optimal postoperative wound healing.
Preoperative Planning
Prior to surgical resection, patients should undergo local and systemic staging studies. Musculoskeletal malignancies have a propensity to pulmonary spread. Therefore, a chest CT is mandatory to screen for systemic disease. An MRI (and plain radiographs for primary bone tumors) is sufficient for gauging the local extent of disease and response to treatment. A CT of the pelvis is often useful to complement the MRI as this area is difficult to image. Surgical planning relies on MRI images taken before and after neoadjuvant therapies. Pretreatment MRI images may be helpful in distinguishing radiation-induced reactive changes from actual tumor tissue.
Most patients with high grade bone malignancies will undergo some form of neoadjuvant treatment, including chemotherapy and/or radiation therapy prior to tumor resection. Typically, primary bone sarcomas such as osteosarcoma are treated with several cycles of preoperative chemotherapy, surgery, and then several additional cycles of chemotherapy. Radiation therapy also has an established role in treatment of soft tissue sarcomas.
The treatment team must choose between pre- and postoperative radiation therapy. Both approaches have advantages and shortcomings. Preoperative treatment requires a smaller area of treatment, creation of a fibrous rind around the tumor, and often causes tumor shrinkage, leading to an improved ability to obtain wide margins without sacrificing vital structures. Preoperative radiation’s disadvantages include a higher rate of wound problems and less viable tumors available for pathologic examination. Postoperative radiation has the advantage of earlier surgery, viable tumors for pathologic study, and fewer wound complications. Treatment volumes however are increased and there is a delay in administering treatment to allow time for adequate healing of operative wounds. We prefer preoperative radiation for pelvic and retroperitoneal sarcomas.
Brachytherapy requires proper reconstruction planning so that flaps do not interfere with catheter placement (Fig. 35-1). Afterloading catheters should be evenly spaced and sutured in place to the tumor bed with fast absorbable sutures to prevent their displacement during postoperative therapy. Alternatively, VAC dressing can be used as a temporary coverage of brachytherapy catheters, followed by delayed primary reconstruction of the defect after completion of brachytherapy. Intraoperative radiation therapy (IORT) is another means of augmenting a preoperative radiation therapy regimen, allowing for directed treatment at close intraoperative margins.
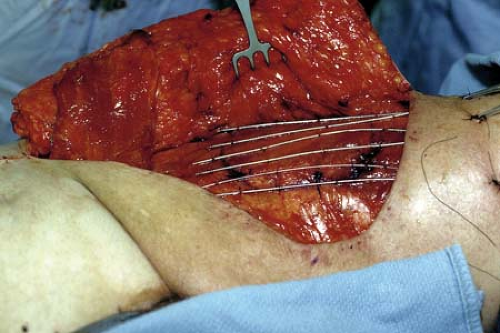 FIGURE 35-1 Placement of afterloading brachytherapy catheters under inferiorly based TRAM flap in treatment of recurrent sarcoma of the thigh. |
Table 35-2. Principles of Pelvic Reconstruction | |
|---|---|
|
Preoperatively, the patients with large pelvic tumor undergo mechanical bowel preparation and intravenous antibiotic coverage. Ostomy sites must be preoperatively marked in accordance with anticipated flap use because inappropriate colostomy or ileostomy placement may burn an important reconstructive bridge and prevent rectus abdominis flap elevation. Involvement of several surgical services such as urological, colorectal, vascular, spine, and plastic surgery is common. The patient is positioned on a bean bag prior to induction of general anesthesia. Large bore intravenous access is established in an event of rapid blood loss. We liberally use ureteral stents that facilitate intraoperative identification of the ureters. After placement of the stents and Foley catheter, the patient is placed in the “sloppy” lateral decubitus position and is secured with the bean bag. This position is preferred for internal or external hemipelvectomy because it permits a wide skin preparation and an easy access to the abdomen, buttock, and perineal regions. If additional procedures on spine, sacrum, or rectum need to be performed, intraoperative repositioning of the patient will be required (Table 35-2).
Low sacral resection can also be performed in the “sloppy” lateral decubitus position (abdominolateral sacral portion) or a full lateral position with the hip and knee joints in 90-degree flexion. When combined abdominal exploration may be required to deal with the intrapelvic anterior component of the tumor, we start the abdominal portion of the operation supine and later reposition the patient for the posterior, sacral stage of the procedure. Plastic surgeon performs an initial marking and flap dissection as dictated by an anticipated defect.
Surgery
External Hemipelvectomy Reconstruction
Pelvic reconstruction following external hemipelvectomy is principally accomplished with three pedicled flap designs: posterior, long anterior, and total thigh fillet flaps. The vast majority of hemipelvectomy defects can be closed with these flaps which constitute the first choice for hemipelvectomy flap reconstructions. If these standard hemipelvectomy flaps are unusable due to very proximal vascular ligation, causing flap ischemia, division of the flap origin during tumor resection or previous procedure, or extensive radiation damage, then alternative flaps must be used for coverage. These second-line reconstructive options include contralateral inferiorly based vertical rectus musculocutaneous (VRAM) flaps, microvascular lower extremity fillet flaps, or standard free flaps depending on the defect
configuration. Likewise, the second-line reconstructive options are useful for closure of hemipelvectomy wounds in the setting of postoperative wound complications (Table 35-3).
configuration. Likewise, the second-line reconstructive options are useful for closure of hemipelvectomy wounds in the setting of postoperative wound complications (Table 35-3).
Table 35-3. Purpose of Soft Tissue Flaps in Tumor Surgery | |
|---|---|
|
Posterior Hemipelvectomy Flap
The classic hemipelvectomy technique relies on pelvic exploration, ligation of the common iliac vessels, division of the pelvic rim by disarticulation of the pubic symphysis and the sacroiliac joint, and creation of the posterior fasciocutaneous flap to achieve soft tissue closure. It was initially recommended that gluteal muscles be left with the specimen. This fasciocutaneous hemipelvectomy flap was based on relatively poor random blood supply due to ipsilateral ligation of the common iliac vessels and was further compromised by removal of the gluteal muscles that greatly increased wound complication rates.
Three modifications of this classic technique aimed to decrease high wound complication rates:
Incorporation of gluteal muscles in the hemipelvectomy flap
Whenever oncologically appropriate, ligation at the level of external iliac vessels with reservation of the internal iliac vessels to improve the flap blood supply
Limited resection of the bony pelvis that allows preservation of the sacral perforators
With these modifications, the posterior hemipelvectomy flap is designed as a musculocutaneous flap based on the superior and inferior gluteal vessels (Fig. 35-2). Preservation of the gluteal muscle decreases posterior flap necrosis rates and makes the construction of a long, viable posterior flap that would reach up to or above the level of umbilicus possible. Impact of the level of vascular ligation on hemipelvectomy wound outcomes has been a point of controversy. Several reports from Karakousis et al suggested that the level of vascular ligation does not affect the posterior hemipelvectomy flap viability and the rate of postoperative wound complications. These authors believed that there was an adequate blood supply of the gluteal muscle through small arterial branches along its sacral origin, which was sufficient to sustain the viability of the flap unless resection of the edge of the sacrum was oncologically necessary. In our experience, we found 2.7-fold higher rates of posterior hemipelvectomy flap necrosis in the patients that had ligation at the level of common iliac vessels. This finding was independent from sacral resection performed during extended hemipelvectomy in some of these patients (1).
Long Anterior and Total Thigh Fillet Hemipelvectomy Flaps
One of the major limitations of the posterior flap external hemipelvectomy is its inability to deal with the advanced tumors of the buttock and posterior pelvis in an oncologically sound manner. In 1953, Bowden et al described utilization of the skin of the femoral triangle based on the preserved segment of the superficial femoral artery for closure of the hemipelvectomy performed for the sarcoma of the buttock. However, it was the critical need for soft tissue reconstruction of the advanced decubiti and the infection of bony pelvis in paraplegic patients that led to increased utilization of the soft tissue obtained from high amputations. The total thigh flap was proposed by Georgiade et al as a last-resort reconstructive option for such patients in the 1950s and subsequently gained wide-spread use. This principle was subsequently applied for coverage of the hemipelvectomy defects whereby a musculocutaneous flap of the anterior thigh compartment was elevated based on the superficial femoral artery. The technique was further refined by Sugarbaker et al, who also demonstrated that the anterior flap can be used as a sensate island flap based on the superficial femoral vessels and saphenous nerve.
Standard long anterior flap hemipelvectomy includes the bulk of quadriceps femoris muscle (Fig. 35-3). A total thigh fillet flap utilizing the majority of the thigh musculature can also be designed as a variation of the anterior hemipelvectomy flap technique (Fig. 35-4). Anterior hemipelvectomy flap is an axial pattern musculocutaneous flap based on the branches of femoral vessels, including lateral




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








