Principles of revision cervical spine surgery are based on adequate decompression of neural elements and mechanical stability via appropriate selection of surgical approach and constructs producing long-term stability with arthrodesis. When planning revision surgery, the surgeon must consider the cause of the underlying problem (eg, biological, mechanical), the potential for complications, and clinical outcomes that can reasonably be expected. This information should be clearly explained to the patient during the informed consent process. This article provides the spine care provider with an understanding of how to appropriately evaluate and manage the most common cervical conditions that require revision cervical spine surgery.
Revision cervical spine surgery can be a complex and risky endeavor. The indications for revision surgery are numerous and include pseudarthrosis, infection, adjacent segment disease, same segment disease, instrumentation failure, and progressive deformity. The evaluation, diagnosis, and management of each of these problems can be challenging. It is essential that the underlying problem be identified through a comprehensive history taking and physical examination as well as appropriate imaging studies. It is also essential to understand why the initial procedure failed so that a similar situation can be avoided during revision surgery. When planning revision surgery, the surgeon must consider the cause of the underlying problem (eg, biological, mechanical, and so forth), the potential for complications, and clinical outcomes that can reasonably be expected. This information should be clearly explained to the patient during the informed consent process. This article provides the spine care provider with an understanding of how to appropriately evaluate and manage the most common cervical conditions that require revision cervical spine surgery.
Considerations for revision surgery
History Taking
Patient history and examination are essential to determining whether or not a patient is a candidate for revision cervical spine surgery. History taking should include a thorough discussion of the initial procedure. Questions that should be asked include the following: why did you have your initial procedure; what symptoms were you having before your initial procedure; following the initial procedure, did you get relief from some or all of your symptoms; if so, how long did this relief last; are the symptoms you are having now similar to those you had before your initial procedure; and if not, how are the symptoms different. These questions will give the spine care provider some sense of whether the initial problem was successfully treated and whether the current symptoms represent persistence of the initial problem, recurrence of the initial problem, or a new problem at an adjacent level. Questions regarding constitutional symptoms (ie, fever, chills, nausea, vomiting, unexplained weight loss, fatigue) should also be addressed during the history taking to assess for problems such as infection or tumor. Questions pertinent to the nature, duration, severity, and location of pain, numbness, and/or tingling as well as questions relating to weakness, problems with balance and fine motor skills, and bowel and bladder function are essential as they are when assessing any spine patient. Red flags such as progressive weakness, constitutional symptoms, and unrelenting pain are suggestive of an urgent or even emergent situation. The patient should also be asked about hoarseness and/or swallowing problems that may be attributed to the initial procedure and may affect the surgical approach for the current problem.
Physical Examination
Whether a patient presents for primary or recurrent problem, a thorough physical examination is indicated that includes inspection, palpation, range of motion test, a full neurologic evaluation, and provocative tests specific to the cervical spine. It is not uncommon that shoulder, elbow, or wrist pathology can mimic cervical spine pathology. Such pathologic conditions must be ruled out during the patient evaluation to avoid unnecessary revision cervical spine surgery. This is particularly true when the initial procedure did not provide any relief of the patient’s original symptoms, suggesting that pathologic condition of the upper extremity rather than the cervical may be the cause of the original symptoms. On inspection, the location and appearance of the initial incision should be noted. Erythema, incisional drainage, and incisional tenderness may indicate the presence of infection. The side of the incision is particularly important when performing anterior cervical surgery because it oftentimes dictates the side of the approach during revision surgery.
Imaging
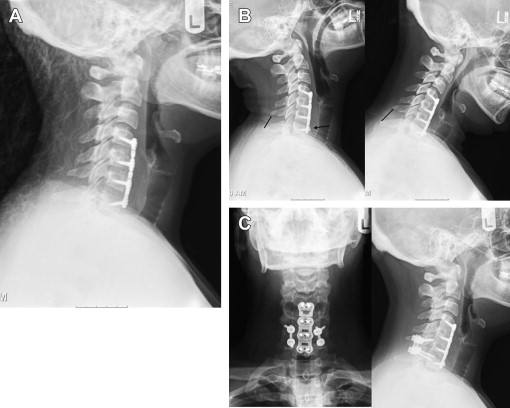
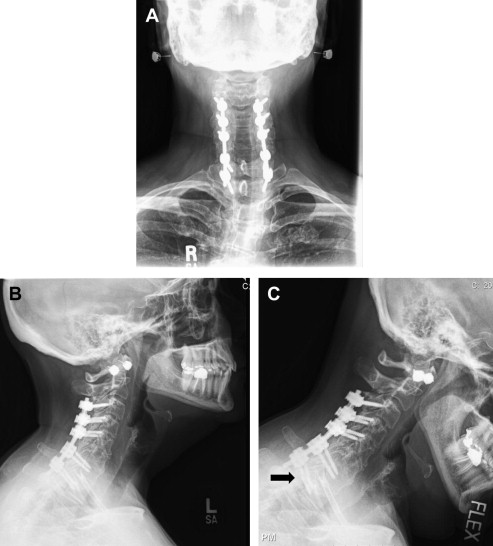
Imaging techniques that are most often used to evaluate a patient for revision cervical spine surgery include plain radiography, computed tomographic (CT) scan, and magnetic resonance imaging (MRI). Plain radiography should typically include anteroposterior, lateral, and flexion/extension views. Cervical alignment (ie, loss of lordosis, kyphosis) should be measured on the lateral radiograph. The status of an existing fusion should be assessed, looking for the presence of bridging trabecular bone or continued motion. The presence and location of instrumentation should be noted. Subtle loosing of existing screws in the form of haloing can indicate pseudarthrosis ( Fig. 1 ). Implant failure in the form of screw pullout and screw and/or rod breakage should be noted. Catastrophic failure of implants can be seen in the setting of spinal instability (ie, from trauma, tumor, or infection) or deformity treated with an instrumentation construct that provides inadequate biomechanical stabilization. Infection and pseudarthrosis should also be considered when subtle or overt signs of instrumentation loosening or failure are noted on radiographs. Flexion and extension lateral cervical radiographs are helpful in assessing for pseudarthrosis and instability. Excessive motion (ie, >2-mm difference, measured between the spinous processes on flexion and extension radiographs) suggests that the fusion, often an anterior cervical diskectomy and fusion (ACDF), is not fully healed. Flexion and extension radiographs may also show movement of loosening screws and angular motion that also suggests pseudarthrosis and/or instability ( Fig. 2 ). Instrumented posterior cervical fusions that develop pseudarthrosis may not move on flexion and extension but may rather show screw breakage or loosening.
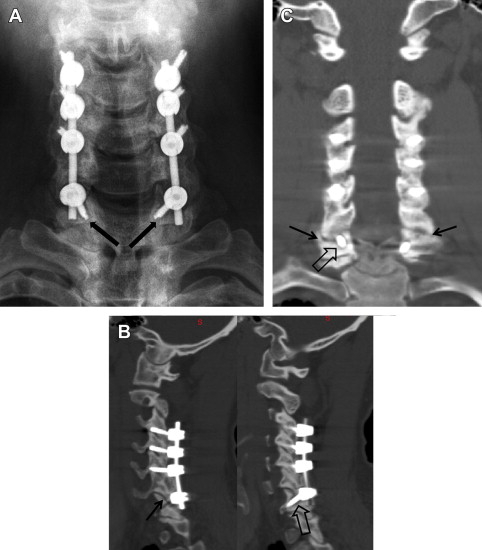
CT scan is helpful in identifying uncovertebral osteophytes and neuroforaminal narrowing in the setting of same segment or adjacent segment disease. CT scan is commonly used as the imaging modality of choice to assess patients with previous cervical fusion and possible pseudarthrosis. Coronal and sagittal reconstructions are particularly helpful in assessing the fusion as well as the position and status of the instrumentation. Anterior cervical fusion after corpectomy and/or diskectomy and posterior cervical fusion between the lateral masses and facet joints can be assessed with great detail using CT scan. Bridging trabecular bone in these areas indicate a solid fusion. Lucency that is typically linear in nature indicates a pseudarthrosis. Lytic bony destruction may be seen in cases of infection or tumor. Loosening of the instrumentation in the form of haloing and/or overt screw pullout can be noted. Screw and rod breakage may or may not be noted on CT scan and, in many cases, is better assessed during plain radiography (ie, static and dynamic studies) ( Fig. 3 ). CT scan can also show in detail bony destruction because of loose instrumentation, infection, and/or tumor. The status of the bone should be noted, particularly if revision instrumentation is planned. Significant bony destruction precludes the placement of instrumentation and can alter the surgical plan, oftentimes leading to proximal and/or distal extension of the instrumentation into areas of preserved bony anatomy.
MRI provides great detail of the soft tissue structures of the cervical spine, including the intervertebral disk, interspinous ligaments, and neural structures. It is used to aid in the diagnosis of same segment and adjacent segment disease, spinal cord compression, infection, and tumor. Postoperatively, MRI is helpful in identifying deep epidural fluid collections that can represent infection. In patients who have had prior cervical surgery, MRI of the cervical spine can be performed with and without gadolinium. This helps differentiate recurrent disease and/or fluid collections from scar tissue. Scar tissue is vascular and therefore enhances after the intravenous administration of gadolinium, which has a high signal intensity on the T1-weighted image. Herniated intervertebral disks and fluid collections; however, are avascular and generally have a low signal intensity on the T1-weighted image. In patients who cannot have an MRI, CT myelography is helpful when assessing the neurologic structures for evidence of compression.
Additional Testing
Certain laboratory tests can be helpful in working up a patient for revision cervical spine surgery. If there is a concern that the patient may have an infection, a complete white blood cell count with differentiation, an erythrocyte sedimentation rate (ESR), and determination of C-reactive protein (CRP) level should be recommended. Elevation of 1 or all of these markers should raise suspicion of infection. ESR and CRP are both nonspecific markers of inflammation. CRP is a protein that is made by hepatocytes in response to inflammation (ie, cytokines). The normal concentration of this protein is 10 mg/L. Postoperative infection can lead to elevation of the CRP level from 40 mg/L to more than 200 mg/L. Units for CRP levels vary amongst laboratories, with some reporting as milligrams per deciliter. The ESR is also a measure of inflammation, with a normal ESR considered to be less than 15 to 20 mm/h. In response to inflammation, CRP level is elevated more rapidly than ESR. Similarly, after the source of inflammation is successfully treated, CRP level returns to normal more rapidly than ESR, with a reported elimination half ranging from 2.6 to 9 days. Kahn and colleagues reported that, in the setting of postoperative spinal wound infection, CRP level is of value in following the response to treatment but that ESR can remain persistently elevated, despite a normal CRP level and clinical evidence of a successfully treated infection. Both the CRP level and ESR are normally elevated after surgery. In one study, after uncomplicated spine surgery, the CRP level and ESRs were noted to peak at 166 mg/L and 68 mm/h on postoperative days 2.7 and 4.2, respectively. Unless a wound infection develops, the CRP level should normalize within 1 to 2 weeks and the ESR should normalize within about 6 weeks.
The incidence of recurrent laryngeal nerve injury is reported to be as high as 3.5% in primary and 9.5% in revision anterior cervical spine surgery. When planning revision anterior cervical spine surgery, it is therefore important to send the patient for vocal cord evaluation by an otolaryngologist to determine if there was an injury to the recurrent laryngeal nerve during the initial procedure. Through either a mirror examination and/or direct laryngoscopy performed in the office setting, the otolaryngologist can determine if there is partial or complete paralysis of the vocal cord ipsilateral to the side of the previous anterior approach. If there is evidence of vocal cord paralysis on the side of the previous anterior cervical surgery, the revision anterior procedure should be performed from the same side to avoid injury to the normal vocal cord. If the vocal cords are normal, then it is preferred that the revision anterior surgery be performed from the contralateral side to avoid scar tissue that can complicate the approach.
When planning revision anterior cervical spine surgery, particularly when anterior instrumentation is present, attention must be given to the esophagus. Although esophageal injury is rare, it is a devastating complication. Esophageal injury can occur either as a result of erosion of existing anterior instrumentation through the esophageal wall or as a result of intraoperative injury. The presence of persistent dysphagia after the initial procedure, prominent anterior instrumentation, and/or anterior instrumentation that is backing out should prompt a preoperative evaluation of the esophagus by an otolaryngologist. A videofluoroscopic swallowing study can assess esophageal function during swallowing and can identify adhesion and possibly perforation at the site of existing instrumentation. Direct esophagoscopy can identify whether or not the instrumentation has eroded through the esophagus. When the preoperative evaluation suggests that the existing anterior instrumentation has compromised the esophageal wall, the otolaryngologist should be consulted for intraoperative assistance during the revision procedure. The risk of intraoperative esophageal injury is elevated in the revision setting. Intraoperative assessment of the integrity of the esophagus can be accomplished using methylene blue. An orogastric tube is placed down the esophagus so that the tip of the tube is within the esophagus at the level of the surgery. The surgeon then applies slight pressure to the esophagus distal to the tube and has the anesthesiologist inject 60 mL of methylene blue/saline. After the solution is injected, the anterior cervical wound is assessed for the presence of the blue solution, the presence of which suggests an esophageal injury.
Spinal pathology requiring revision surgery
A comprehensive systematic approach is required when a patient presents with recurrent symptoms after prior cervical spine surgery. Surgery performed at the wrong level, gross hardware failure, or instability often requires revision surgery. The presenting signs and symptoms (axial pain, myelopathy, or radiculopathy) guide diagnostic workup and management. Workup of axial neck pain after fusion is best accomplished with CT scan with sagittal and coronal reconstructions to determine the presence or absence of fusion. If symptomatic pseudarthrosis is present, revision surgery may be indicated. If solid fusion is noted, MRI can identify the presence of same and/or adjacent segment degenerative disease, which may be managed conservatively or surgically. CT myelogram and/or MRI can typically identify same or adjacent segment central or foraminal stenosis. Patients with myelopathy typically require revision decompression, whereas those with radiculopathy can be managed nonoperatively or operatively. If MRI is unremarkable, electromyography (EMG) and nerve conduction studies (NCS) are indicated to evaluate for peripheral nerve compression or polyradiculopathies.
Adjacent Segment Disease
Adjacent segment cervical disease (ASD) is noted in roughly 3% of patients per year; however, incidence increases to approximately 25% of patients within the first 10 years after the index fusion procedure is expected. Adjacent segment disease, simply defined, is the development of symptomatic spondylosis and/or disk herniation adjacent to a prior fused level. Presence of clinical symptoms delineates this definition from adjacent segment degeneration, which refers to the presence of degeneration on imaging studies. A host of clinical, biomechanical, and basic science reports abound in the literature regarding the cause, nature, and course of the disease. Pivotal to the debate is the question whether degeneration occurs as a result of the natural history in patients at risk for spondylosis or the increased motion and stress at the disc adjacent to a rigid fusion. A combination of factors probably contributes to ASD development, including the increased biomechanical stress placed on the disk space adjacent to a fusion and the natural history of cervical spondylosis in patients known to have such pathology.
Clinical studies primarily concentrate on retrospective reviews with varying imaging modalities to determine adjacent segment degeneration with or without clinical symptoms. Dohler and colleagues found an asymptomatic adjacent level translation of 67% at 27 months of mean follow-up after ACDF. Kyphotic alignment at an operated level might be a risk factor for adjacent segment degeneration. Katsuura and colleagues failed to show differences in Japanese Orthopaedic Association scores before, after, or at final follow-up in patients with or without adjacent segment degeneration. At a long-term 100-month follow-up, Goffin and colleagues showed an incidence of adjacent segment degeneration of 92% correlated poorly with ASD and reoperation (6%). Hilibrand and colleagues noted an annual incidence of ASD of 2.9% with 25% chance at 10-year follow-up. Other studies corroborate these findings and found increased risk of symptomatic ASD when index operation preoperative imaging showed ventral dural compression via the disk, lending some to recommend inclusion of the adjacent levels at the index operation.
In response to increased awareness of ASD, motion-sparing techniques and devices have been advocated, primarily including posterior laminoforaminotomy and cervical disk replacement. Although most studies of ASD pertain to anterior cervical fusion, posterior procedures also experience ASD and degeneration postoperatively. Laminoforaminotomy has been implicated with similar rates of adjacent segment pathology compared with ACDF (50% vs 41% at 4.5-year follow-up) in addition to 3.3% same segment disease at 7-year follow-up. This suggests that natural history likely contributes to ASD development after posterior laminoforaminotomy. Clinical studies have varied in results when comparing adjacent segmental motion after arthroplasty with ACDF, with some showing increased motion, no difference, or decreased motion in patients with arthroplasty. Clinical outcomes seem to be similar functionally between arthroplasty and single-level ACDF with allograft and plating, although the need for reoperation due to ASD might be lower with arthroplasty.
Presenting symptoms and signs of ASD may include myelopathy- and/or radiculopathy-related complaints. Static and dynamic radiographs may demonstrate changes of spondylosis or degeneration, including disk space narrowing; subchondral end plate sclerosis; osteophyte (anterior, posterior, or uncovertebral) formation; and, less commonly, instability. MRI is the modality of choice to evaluate the discoligamentous structures, when assessing for neural compression correlating with the patient’s clinical presentation.
Same Segment Disease (Incomplete ACDF or Disk Arthroplasty)
Same segment disease usually implies inadequate decompression at the levels operated at the index procedure or, in the setting of cervical disk replacement, the recurrence of disease. Revision anterior surgery for inadequate decompression is typically more difficult than the original procedure, including dissection through a scar bed with and takedown of prior hardware and bone graft material. To adequately diagnose and treat same segment disease, the physician must be completely versed in the differential diagnoses mimicking compressive cervical lesions. Once an accurate diagnosis is made, use of imaging studies, including static and dynamic radiography, CT, and MRI (occasionally including dynamic studies and oblique views to evaluate for more subtle dynamic compression or foraminal disease), can allow the surgeon to create acomprehensive preoperative plan based on the location of compression, number of levels involved, presence of hard or soft disks, sagittal alignment, presence of retrovertebral pathology or ossification of the posterior longitudinal ligament (PLL), presence of instability, and axial neck pain. The surgeon’s armamentarium of anterior (ACDF, anterior cervical corpectomy and fusion [ACCF], hybrid ACCF + ACDF), posterior (laminoforaminotomy, laminectomy with fusion, or laminaplasty), or combined procedures can then be appropriately used.
In patients who present with persistence of preoperative symptoms, the treating physician must consider the possible causes, including inaccurate original diagnosis, inadequate decompression, unrecognized preexisting irreversible spinal cord/nerve root, iatrogenic spinal cord/nerve root injury, postoperative instability, adjacent segment disease, and pseudarthrosis. Differential diagnosis for cervical myelopathy includes intracranial, intraspinal, and peripheral nerve disorders such as multiple sclerosis (visual symptoms, facial numbness, migratory neurologic complaints, oligoclonal cerebrospinal fluid [CSF] bands); amyotrophic lateral sclerosis (combined progressive upper and lower motor neuron disease of anterior horn cells); Guillain-Barré syndrome (increased CSF protein in postviral patients with ascending sensorimotor polyneuropathy); pernicious anemia (vitamin B12 deficiency with macrocytic anemia, dorsal column dysfunction); peripheral nerve entrapment disorders of the radial, median, and ulnar nerves (commonly carpal and cubital tunnel syndromes); syringomyelia; irreversible preexisting spinal cord changes; infection; or intraspinal or intracranial tumors. Workup is dictated by thorough history taking, physical examination, and then subsequent imaging (spinal or intracranial CT with/without myelography, MRI) or diagnostic tests (EMG/NCS, CSF analysis, laboratory analysis, and consultation with neurologists as indicated).
CT myelography is excellent for evaluating adequacy of decompression, presence of fusion or pseudarthrosis, graft and hardware location and status, presence of residual bony compression, infolding of redundant ligamentum flavum, and facet arthrosis. MRI can evaluate the status of the spinal cord and quantify myelomalacia and cord atrophy and whether reversible (low-intensity T2 changes) or irreversible (high-intensity focal T2 and low-signal T1 changes or “snake-eye” appearance of anterior horn cells cystic necrosis) changes are present. In addition to these MRI signal changes, chronic symptoms for more than 18 months, age more than 60 years, spinal cord transverse area less than 50 mm 2 , and multilevel compression are risk factors for poor outcomes. Intraoperative neurologic injury should be noted in the initial records on transcranial motor evoked potentials and somatosensory potentials secondary typically to direct neurologic trauma or hypoperfusion from hypotension.
After defining the offending structures, surgical plan and procedures are chosen based on multiple factors. If compression is located anteriorly, presence of 1- or 2-level disease is best approached with ACDF if any spondylotic or hard disk component is noted with excellent results. If the PLL had not been previously resected, it is recommended in the revision setting to resect the PLL and perform a direct uncovertebral decompression to ensure that the compressive pathology is adequately removed. Patients with multilevel anterior compression with kyphotic alignment must be evaluated for the need to perform a corpectomy versus multilevel ACDF. Multilevel ACDFs provide increased biomechanical stability and increased lordosis but higher risk of pseudarthrosis given more fusion surfaces (ie, up to 47%–56% with 3- and 4-level cases). Multilevel ACCF has relatively high fusion rates (ie, 86%–99%) but an increased rate of graft dislodgement that usually necessitates posterior fixation for more than 2-level corpectomies. Patients with multilevel (>3 levels) anterior fusions may require posterior supplementary fixation. Compressive lesions located ventrally over multilevels that have neutral or lordotic sagittal alignment can be approached by either laminectomy with fusion (ie, if preexisting instability or axial neck pain is noted) or laminoplasty with or without foraminotomies (ie, when no instability or neck pain exists preoperatively) with good results more than 90% time in appropriately selected patients.
The advent of minimally invasive and motion-sparing technologies does not change the primary need for adequate decompression. Compared with ACDF in which posterior osteophytes may be absorbed over time, patients who have undergone cervical disk replacement require a complete osteophyte decompression because motion is preserved. Careful attention to detail is also required during foraminotomy. Lehman and Riew recommend dorsally unroofing the nerve by resecting up to 50% of the superior articular facet with or without removal of underlying disks or burring of the uncovertebral joint osteophytes. Given the preservation of motion, osteophytes and compressive pathologic condition can reoccur after cervical disk replacement at the same segment. Diagnosis is complicated by the presence of the prosthesis, which creates artifact on imaging studies. CT myelography can be helpful when trying to diagnose same segment disease after cervical disk replacement. Selective nerve root injections can aid in the diagnosis if questions remain as to which levels are symptomatic. The presence of persistent or recurrent foraminal disease after cervical disk arthroplasty may be amenable to posterior laminoforaminotomy.
Pseudarthrosis
Cervical pseudarthroses are known complications of both anterior and posterior fusions and typically occurring at the caudalmost level. Incidence of pseudarthrosis after single-level ACDF ranges from 0% to 20%, escalating to 40% to 50% for multilevel procedures. Cervical spine fusion rates vary depending on many factors, including approach (anterior vs posterior), number of levels fused, type of grafting technique (autograft, allograft), presence of deformity, and patient-specific factors (smoking, diabetes, prior history of pseudarthrosis). Planning revision surgery can be complicated by the presence of prior approach-related complications (ie, anterior scarring, recurrent laryngeal nerve injury, and dysphagia). Patients are symptomatic in two-thirds of pseudarthroses, and patients typically complain of axial neck pain, radiculitis, or a combination of recurring or persisting preoperative symptoms. Dynamic flexion and extension radiographs may show gross instability at the level of pseudarthrosis, screw/rod breakage, migration of instrumentation, and/or haloing of screws. CT scan is the imaging modality of choice when evaluating for presence of pseudarthroses, the status of any instrumentation (ie, loosening, breakage, migration), bone quality, and presence of any offending compressive structures in patients with neurologic symptoms and signs ( Fig. 4 ). Intraoperative confirmation of motion at the motion segment, lack of bony bridging trabeculae, and graft resorbtions are suggestive of a pseudarthrosis.
Treatment of pseudarthroses is usually nonoperative in asymptomatic individuals, and up to 30% do not require revision surgery. Operative treatment is required for those with unstable/failed hardware and appropriate for those select patients who remain symptomatic despite conservative measures. Treatment of posterior pseudarthrosis can be complicated in the setting of prior decompression because there is an increased risk of incidental durotomy during surgical dissection. In these cases, anterior cervical fusion is preferred. Patients without exposed dura can be approached dorsally, ventrally, or circumferentially depending on the amount of stabilization required to achieve a biomechanically sound construct allowing arthrodesis. Treatment options for anterior pseudarthroses typically involve revision anterior repair, posterior fusion, or combined anterior/posterior stabilization and fusion. Direct evaluation, removal, and grafting of the pseudarthrosis are possible via revision anterior repair, but difficult dissection through scar tissue beds increases risk of viscus, vessel, or nerve injury as well as increased rates of dysphagia and dysphonia. Anterior repair typically has lower blood loss and shorter hospital stays but lower union rates (45% vs 95%–97%) than posterior procedures. Overall, complication rates (mostly wound or graft harvest related) are reported to be slightly higher with posterior surgery.
Surgical technique for successful revision anterior repair or posterior fusion hinges on adequate decompression of neural structures, preparation of a good fusion bed, use of adequate bone graft and/or bone graft substitute, and stable fixation. During revision anterior repair, complete excision of fibrous nonosseous material is required as well as a thorough decompression of the neural elements, especially in patients with signs and/or symptoms of radiculopathy or myelopathy. Iliac crest autograft is a reliable option for grafting in the revision situation. Posterior fusion alone or as part of circumferential stabilization is the authors’ treatment of choice as long as anterior hardware and grafts are stable and kyphotic deformity does not exist. This approach provides a high rate of fusion and clinical success, more so than the anterior approach. Decompression in most cases consists of laminoforaminotomy at the indicated levels. Kuhns and colleagues demonstrated no difference in patients treated with autogenous iliac crest or local bone graft. Stabilization is typically achieved with posterior lateral mass screw/rod constructs. At the C7 and T1 levels, the authors typically use pedicle screw fixation (see Fig. 2 C).
Progressive Deformity (Postlaminectomy Kyphosis)
Iatrogenic cervical deformity most often occurs after posterior decompressive procedures involving disruption of the tension band (posterior ligamentous complex), muscular denervation and weakness, and excessive capsulectomy and/or facetectomy. Although the deformities can involve both the coronal and sagittal planes, the dominant alignment is typically kyphosis because the center of gravity shifts anteriorly given the weight of the head, muscular weakness, and ligamentous and articular disruptions. Multilevel cervical laminectomies carry a 20% risk, which can be higher in skeletally immature and young adult patients (given incomplete osseous formation, wedging, and relative ligamentous laxity). Iatrogenic kyphosis is also increased after laminoplasty secondary to the disruption of the muscular, fascial, and ligamentous structures. Risk factors include increased age, preoperative sagittal kyphotic alignment, poor intraoperative positioning, inadequate grafting techniques or subsidence, and aggressive facetectomies. Clinically, patients typically present with muscular fatigue, neck pain, difficulty maintaining a horizontal gaze, a kyphotic sagittal appearance on lateral observation with the head protruding forward, and possible neurologic symptoms and signs, including myelopathy or radiculopathy. Diagnostic workup radiographs help evaluate coronal imbalance, maximum deformity, and the amount of correction that can be obtained (fixed vs flexible deformity). CT scan is helpful in determining the presence of bony ankylosis dorsally or ventral fusion in patients with fixed deformities as well as in planning surgical corrective maneuvers ( Fig. 5 ). MRI is useful for patients with neurologic complaints or signs to determine where decompression is needed as well as to evaluate for and quantify the amount of myelomalacia, cord atrophy, and syringomyelia, which can complicate as well as increase risk of treatment.








