Fig. 14.1
Echographic image of mild focal soleus muscle overstretching
US and MRI are equally sensitive in assessing muscle or tendon injury. However, MRI offers a more detailed analysis of the lesion, and it is not user dependent (Fig. 14.2).
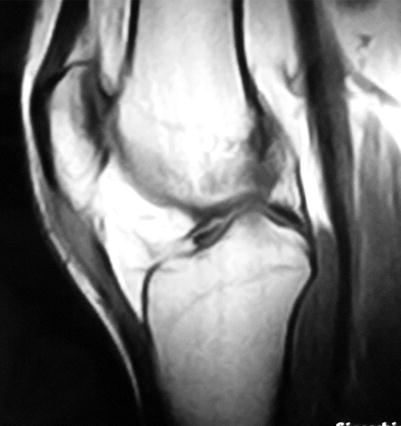

Fig. 14.2
MR image of insertional patellar tendinopathy
Despite all these features, there is no clinical classification system able to predict the return to play on the basis of the mere extent of the lesion seen on MRI [2].
Nevertheless, several studies have demonstrated that whenever a structural damage is identified on MRI, return to play will take longer than those traumas without any structural defect [14].
Some authors have reported MRI findings at return to sport, such as still increased signal intensity on fluid-sensitive sequences (correlated with edema persistence) or decreased signal intensity (correlated with scar tissue formation) [15, 16].
However, a high intensity signal in fluid-sensitive sequences in MRI sometimes does not indicate the specific phase of injury. There are indeed clinically recovered athletes in which the amount of increased signal intensity in fluid-sensitive sequences at return to sport exceeds that of other athletes at the time of initial injury. This seems to suggest that the extent of this kind of sequences does not differentiate an injured from a recovered muscle [17, 13, 12].
Nevertheless changes of the hematoma over time can successfully guide treatment decisions and managing [10].
Tendon Doppler flow among active athletes can show adaptive response to mechanical loading. A neovascularity is considered one of the hallmark features of tendinopathy. Despite this, it has not been demonstrated whether tendon Doppler flow is related to prolonged activity, although it is well known that Doppler flow increases after an acute improvement of mechanical loading [18].
Some authors have established a relationship between Doppler flow and tendon structure abnormalities, in particular the tendon was characterized by a widening of the structure and the presence of an hypoechoic region [19]. Conversely, other authors have demonstrated that there was no association between Doppler flow and hypoechogenicity, but between Doppler flow and heterogeneous echogenicity, which may explain the lack of correlation between Doppler flow and pain [18].
A unique correlation between Doppler flow imaging and pathological disorders does not exist yet, because it depends on multiple factors including athlete’s activity level, the amount of mechanical loading, and operator’s ability.
Taking together several studies, it can be stated that Doppler flow is not necessarily a sign of current tendon pain, but it can be associated with tendon stiffness or pathologic abnormalities (Fig. 14.3).
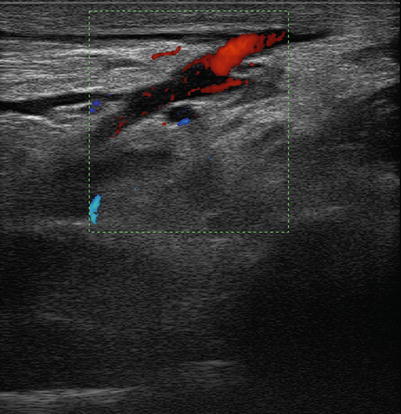

Fig. 14.3
Distal biceps tendon detachment. Echographic image
Therefore, Doppler flow can be correlated to asymptomatic changes in tendon morphologic characteristics in response to mechanical load among active athletes [18].
Generally speaking, several researches have affirmed that tendons showing US signs of tendinopathy can be pain-free, and at the same time, US alterations are not necessarily associated with bad prognosis [20].
It can also be said that sensory nerves probably contribute to produce pain in the site of increased vascularity. Even biochemical substances like substance P or glutamate, correlated with neurovascular ingrowth, can be involved in the development of tendon pain [21].
It seems plausible that tendons undergo changes during the asymptomatic phase before the pain threshold is reached and the alterations become symptomatic [22].
However, recent studies point out that intratendinous flow can be increased during exercise both in normal healthy asymptomatic patients and in symptomatic athletes.
This suggests that intratendinous flow is not necessarily a sign of overuse injury, but it could be just a normal physiological response to loading [23].
Therefore, it should be underlined that US and color Doppler must be associated to other diagnostic tools, such as MRI and clinical tests, to provide a complete evaluation.
Most muscle injuries successfully respond to conservative management.
A short period of total inactivity is fundamental for a rapid and complete recovery. However, immobilization should not last more than 1 week, so that the adverse effects of immobilization can be limited. The ideal resting period should last 4–6 days after injury, preventing excessive scar formation and reinjuries at the lesion site [24].
This initial rest must be followed by active rehabilitation, as an early mobilization intensifies the regeneration phase as well as it induces angiogenesis. Exercise also facilitates the regenerating myofibers to arrange themselves in the proper orientation [24].
Caution is fundamental when the repair site remains completely or even partially unloaded for a while.
Whereas some kind of structures show benefits from immobilization, like rotator cuff tendons, other tissues like flexor tendons of the hand or patellar tendon can develop fibrous adhesions and a reduction in repair strength, probably due to increased collagen turnover rather than atrophy.
A complete and prolonged removal of load has been demonstrated to limit the healing process of muscles or tendons, largely due to a decreased production of extracellular matrix.
These concepts can be also applied when passive motion is required. Some tendons need long excursions to restore the proper functioning, because they are encased in synovial sheaths. Therefore, in order to maintain the proper tissue sliding, adhesions between the tendon surface and its sheath must be prevented.
At any rate, whatever the tendon-specific function is, continuous passive motion after immobilization or/and after surgery can only be helpful for a complete tendon or muscle healing [25].
Unfortunately, we cannot rely on specific measures for predicting exactly the downtime of the athlete after a serious muscle injury.
It has been supposed by some authors that four simple measures performed during the clinical examination 3–5 days after injury could predict a recovery longer than 4 weeks; these parameters take account of the presence of bruising or hematoma, tenderness to palpation, lack of complete ROM, and pain during isometric limb lengthening [26].
It has been widely demonstrated indeed that athletes who take more than 1 day to walk painless after injury will need more than 3 weeks to return to competition. At the same time, a reduction of ROM worse than 30° determines a prolonged layoff period after injury [24].
Another important healing goal is to take account of all those modifiable risk factors which can induce injury recurrence. These are muscle weakness, fatigue and lack of flexibility, with a strength disparity between eccentric and concentric muscles. As a result, a good rehabilitation program must address all these factors [8].
Rehabilitation protocols vary according to the clinical case, depending on all the information about the event, such as the site, quality, and severity of the injury.
However, some authors have established a protocol valid for track and field activities which is divided into four phases. The first one is the acute phase, during which immobilization is required to normalize the gait. The second one is the range of motion phase, to regain a painless ROM, starting with concentric and progressing to eccentric training. The third, functional one establishes limb loading and returning to running activities, agility drills, and plyometric training. The last stage is the return-to-play phase: it is achieved after a variable time, which depends on the severity of the injury as well as the athlete’s response to the whole protocol [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







