CHAPTER 32 Results and Complications
Reports on the results of reverse shoulder arthroplasty are becoming more common as implantation of this type of shoulder arthroplasty increases. The results vary predominantly by the underlying indication for which the arthroplasty was performed. We have participated in a large European-based study of the reverse prosthesis and continually enroll patients in our own database.1 The results and complications presented in this chapter are drawn from these sources.
RESULTS
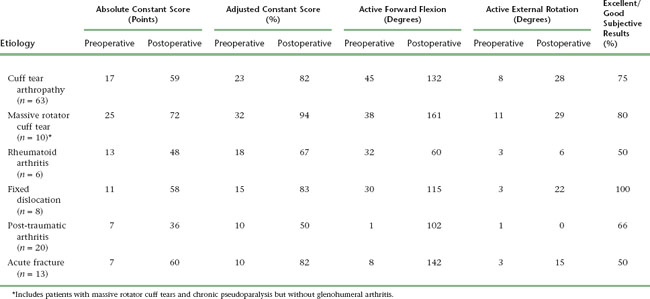
The results of reverse shoulder arthroplasty vary mainly with the etiology for which the arthroplasty was performed. The best results are obtained in the treatment of osteoarthritis with a massive rotator cuff tear (rotator cuff tear arthropathy), whereas results are least satisfactory in patients with post-traumatic arthritis. Tables 32-1 and 32-2 detail the results of reverse shoulder arthroplasty for the most common indications for which it is performed. These tables express the results in terms of active mobility; patient satisfaction; the Constant score, a shoulder-specific outcomes device incorporating pain, mobility, activity, and strength; and the age- and gender-adjusted Constant score.2,3
Table 32-1 RESULTS OF REVERSE SHOULDER ARTHROPLASTY ACCORDING TO UNDERLYING ETIOLOGY IN A LARGE EUROPEAN-BASED STUDY
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
INTRAOPERATIVE COMPLICATIONS
Humerus
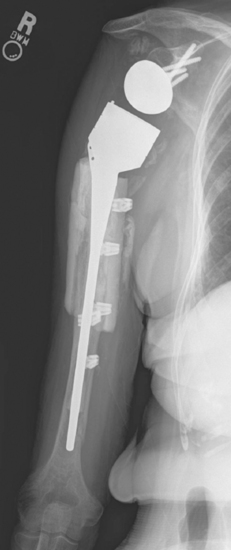
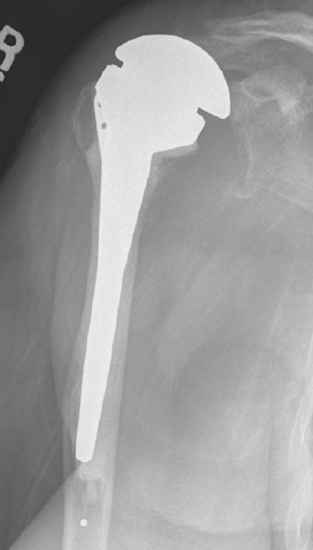
The most common humeral complication is iatrogenic fracture, which usually results from performing an overly aggressive dislocation maneuver without previous adequate soft tissue release. Many patients undergoing reverse shoulder arthroplasty have moderate to severe osteopenia, thus placing them at increased risk for this complication. Intraoperative tuberosity fractures during glenohumeral dislocation are less common during reverse shoulder arthroplasty than during unconstrained shoulder arthroplasty because of the lack of a rotator cuff attaching to the tuberosities, which could otherwise contribute to fracture during a dislocation maneuver. Fractures involving the humeral diaphysis should be reduced and a long-stem humeral implant placed. Allograft struts and cerclage cables may be added in patients with severe osteopenia (Fig. 32-1).
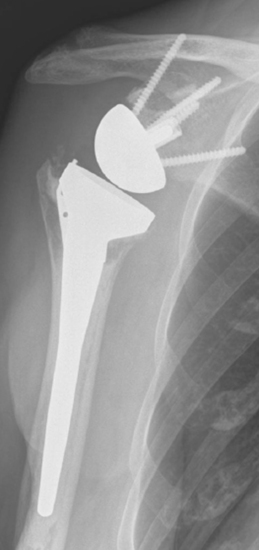
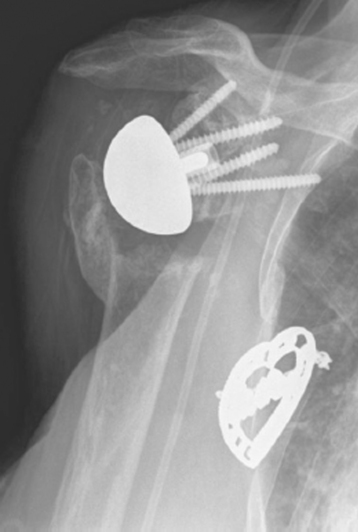
Intraoperative fractures involving the greater or lesser tuberosities (or both) are usually nondisplaced. Many of these fractures are stable or become stable once the humeral implant is inserted (Fig. 32-2). If a greater tuberosity fracture fragment that has maintained attachment of a substantial portion of the posterior rotator cuff is not satisfactorily stable, suture fixation of the tuberosity is performed and the postoperative rehabilitation adjusted accordingly to allow healing of the tuberosity. A greater tuberosity fracture fragment that is devoid of rotator cuff attachment and is unstable may simply be excised.
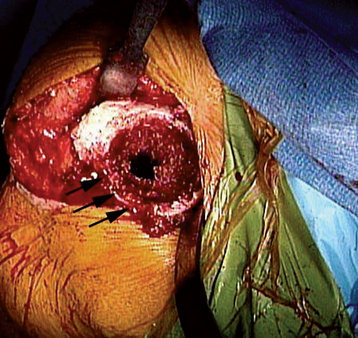
Intraoperative lesser tuberosity fractures commonly occur during retraction of the proximal humerus in the course of glenoid preparation and insertion. The thin anterior cortex of the humeral metaphysis, including the lesser tuberosity, may be crushed by the humeral retractor (Fig. 32-3). Creation of this fracture is often necessary to obtain adequate glenoid exposure during insertion of the reverse prosthesis through a deltopectoral approach. This fracture is without clinical consequence and can largely be ignored.
Glenoid
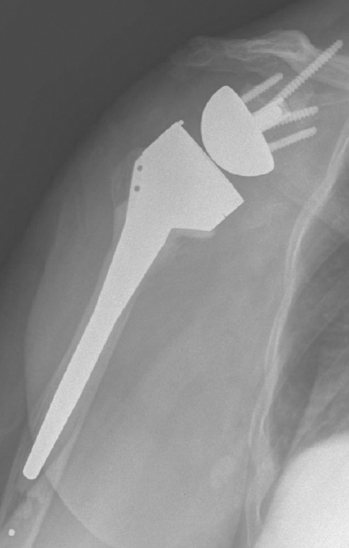
Fractures that involve only a small portion of the peripheral rim generally require no treatment, and the glenoid component can be inserted as planned. Glenoid fractures that extend into the central portion of the glenoid should be bone-grafted with the humeral head. The reverse glenoid component can be inserted to help secure the bone graft and internally fix the fracture. If the central post of the reverse component is firmly seated within native glenoid bone, consideration can be given to placing the humeral component in the same surgical setting. If the glenoid component does not seem secure or the central post of the glenoid base plate is not firmly seated in unfractured native glenoid bone, the humeral component should be initially omitted for 6 months to allow the fracture to heal. After 6 months a humeral component can be placed as the second part of a two-stage procedure (Fig. 32-4). Alternatively, if an intraoperative glenoid fracture occurs, the fracture can be bone-grafted and the humeral stem plus a hemiarthroplasty adapter placed (Fig. 32-5). After the fracture has healed and remodeled (around 6 months after the index attempt at reverse shoulder arthroplasty), the second stage of the procedure, consisting of implantation of the glenoid component, may be performed (Fig. 32-6).
Neurovascular Structures
Catastrophic injury to the neurovascular structures around the shoulder is exceedingly rare during reverse shoulder arthroplasty. The neural structures most at risk during reverse shoulder arthroplasty are the axillary and musculocutaneous nerves. These nerves should not be at risk for transection during primary arthroplasty using accepted operative technique. Neuropraxic injury caused by stretch most commonly involves the axillary nerve, but any nerves within the brachial plexus can be affected. Care should be taken when positioning the patient to maintain the cervical spine in neutral alignment to avoid a stretch injury to the brachial plexus. We have yet to establish risk factors for neuropraxic injury to the axillary nerve. Logic would suggest that patients with the most stiffness creating difficulty in glenoid exposure would be at the highest risk for this type of complication. Our clinical experience has not borne this out, however, and we are currently unable to predict which patients are most likely to suffer this complication. Patient education preoperatively is of paramount importance in dealing with neuropraxia inasmuch as patients are much more accepting if they have heard about the possibility of this complication before surgery. Axillary nerve (and other nerve) neuropraxia is treated by observation, with most patients recovering by 3 to 4 months postoperatively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree