Primary Repair and Nerve Grafting following Complete Nerve Transection in the Hand, Wrist, and Forearm
Matthew E. Hiro
Randy R. Bindra
DEFINITION
Complete transection of a peripheral nerve is defined as interruption of all of the axons within the nerve.
Primary nerve repair is the tension-free reapproximation of severed nerve ends performed within a week of injury.
Delayed primary repair is performed up to 3 weeks from injury when local soft tissue injuries do not permit primary wound closure.
The healing of an injured peripheral nerve is different from the healing of other tissue types.
Injury is followed by an immediate degeneration, followed by incomplete recovery.
Irreversible changes in the motor and sensory end organs make timing of repair critical to achieve useful recovery.
ANATOMY
The anatomy of the peripheral nerve can be simplified by examining its component parts (FIG 1).
Axon. The basic unit of a nerve is composed of a cell body, dendrites, and longer axons.
All axons are surrounded by Schwann cells, which produce the myelin sheath surrounding the axon.
Interruptions in the myelin sheath are referred to as nodes of Ranvier. Impulse propagation is faster in myelinated axons using a process called saltatory conduction, as the depolarization potential “jumps” between nodes.
Myelinated fibers are between 2 and 22 µm in diameter. The larger the fiber, the faster the conduction speeds.
Axonal transport of cytoskeletal elements and neuronal factors is oxygen-dependent. Antegrade transport along the axon occurs at roughly 1 to 4 mm per day. The transport is the rate-limiting step in nerve regeneration.
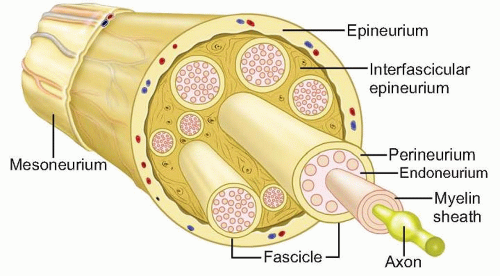
FIG 1 • Schematic of ultrastructure of the nerve. The smallest nerve unit visible to the naked eye is the nerve fascicle.
Endoneurium. Delicate connective tissue that supports and surrounds each axonal fiber and associated Schwann cells
Consists of longitudinally arranged collagen fibrils and intrinsic blood vessels
Perineurium. The connective tissue that surrounds groups of axons, creating bundles referred to as fascicles. The fascicle is the smallest visible unit of the nerve at surgery.
The fascicle is several layers thick and acts as a protective membrane and a barrier to diffusion.
Epineurium. Surrounds groups of fascicles to form the superstructure of a peripheral nerve
Forms a sheath about the entire nerve and also supports the fascicular structure by passing between all the fascicles
Forms 60% to 85% of the cross-sectional area of a peripheral nerve
Composed on longitudinally oriented collagen fibers, fibroblasts, and intrinsic vessels
Paraneurium or mesoneurium. Loose areolar tissue surrounding the epineurium
Limited to the outer surface of the nerve
Location for the extrinsic vascular supply of the nerve
Makes up the gliding apparatus of a peripheral nerve
Fascicles have a definite topographic arrangement within a peripheral nerve.
Fascicular segregation into motor and sensory components is important when aligning a sectioned nerve before primary repair or nerve grafting.
This concept of functional segregation allows for use of part of a donor healthy nerve for nerve transfer with minimal functional deficit.
PATHOGENESIS
Injuries involving peripheral nerves can be simply classified as tidy or untidy.
Tidy wounds involve sharp transections with minimal to no tissue loss:
Sharp lacerations from glass or knife wounds
Most iatrogenic nerve injuries
Untidy wounds involve crushing or avulsion of tissues in the area:
Bony injury may be present.
Surrounding soft tissue may have been lost or rendered nonviable and is expected to heal with significant scarring. This corresponds to the zone of injury.
NATURAL HISTORY
Complete transection of a nerve results in retraction of the nerve ends. The nerve will not heal without surgical intervention to approximate the nerve ends.
Wallerian degeneration occurs in the nerve segment distal to the level of transection.
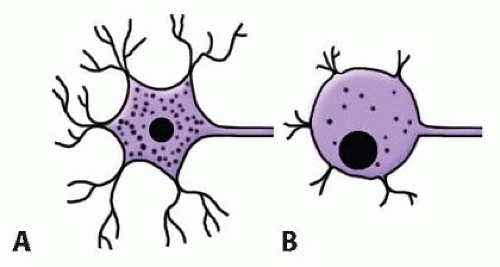
The axon distal to the injury degenerates and does not directly contribute to repair. The axonal and myelin debris are cleared by macrophages. Schwann cells proliferate, releasing nerve growth factors or neurotrophic factors. The distal stump does produce a complex protein, neurotropic factor, that attracts regenerating axons from the proximal stump.
The cell body swells, Nissl granules in the cytoplasm diminish, and its dendritic processes retract. Several cells rupture and die, especially with more proximal nerve injuries (FIG 2).
Regenerating axons sprout from the surviving axons and migrate toward the empty tubules in the degenerate distal stump at a rate of 1 to 3 mm per day.
Proliferating Schwann cells myelinate the newly regenerated axons.
In an unrepaired nerve, the random proliferation of axons from the proximal stump forms a tender mass of disorganized axons and fibrosis termed a neuroma.
PATIENT HISTORY AND PHYSICAL FINDINGS
History of trauma
Penetrating, ballistic, burn, stretch, blunt, fracture, or previous surgery
Timing of onset of symptoms: at initial presentation; after procedure, for example, manipulation and casting or internal fixation of a fracture
Depth and location of the injury
Severity of bleeding-associated blood vessel injury
Patient reports
Paresthesias (pins and needles) or absent sensation (numbness) in fingers
Weakness
Paralysis due to nerve or associated tendon injury
Pain: neurogenic type; can be constant and severe
Rarely, a sensation of warmth or anhydrosis
Physical examination
Note the distribution of sensory loss. The area of sensory loss varies with the nerve that is injured (FIG 3).
Examine the skin for trophic changes or dry skin. Dry, warm skin implies sympathetic interruption.
Perform thumb abduction test to check for paralysis of the abductor pollicis brevis from median nerve injury.
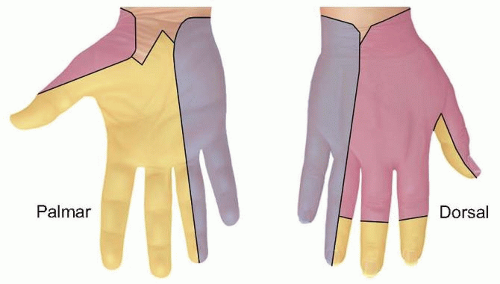
FIG 3 • Distribution of sensory loss with nerve injury. Yellow, median nerve; blue, ulnar nerve; pink, radial nerve.
Perform the Froment sign test. The test is positive if paper is held by flexing the thumb interphalangeal (IP) joint, indicating recruitment of the flexor pollicis longus, which implies paralysis of the adductor pollicis from ulnar nerve injury.
The thumb IP hyperextension test may indicate paralysis of the extensor pollicis longus due to posterior interosseous palsy.
Perform the Tinel sign test. The test is positive if the patient notes a tingling sensation in the sensory distribution of the nerve. Serial progression of Tinel sign distally is useful to monitor axon progression after repair.
When performing physical examinations, it is helpful to use motor function grading according to the Medical Research Council system. This grading allows for qualitative measurement of function and allows the clinician to chart recovery objectively:
M0: no contraction
M1: palpable contraction with only a flicker of motion
M2: movement of the part with gravity eliminated
M3: muscle contraction against gravity
M4: ability to contract against moderate resistance
M5: normal function
Quantitative measurements using grip and pinch strength dynamometers and comparing results to the contralateral normal side may also be useful.
Sensory grading is also useful in evaluation. Sensory function is evaluated within the anatomic distribution of the nerve in question. Sensation is quantified using two complementary tests—(1) Semmes-Weinstein monofilaments, which measure innervation threshold, and (2) two-point discrimination, which measures innervation density. Vibratory, pain, and temperature sensation should also be evaluated. Semmes-Weinstein filaments demonstrate subtle and early sensory loss and are more useful in evaluation of compressive neuropathy. Two-point discrimination measurements help gauge the severity of nerve injury, with two-point discrimination of less than 12 mm indicating neurapraxic injury and readings greater than 15 mm suggesting complete disruption. Used together, the various sensory tests allow for qualitative measurement of function and allow for the clinician to objectively chart recovery:
S0: lack of sensation
S1: recovery of deep cutaneous pain sensibility within the autonomous area of the nerve
S2: return of some degree of superficial cutaneous pain and tactile sensibility
S3: return of function (S2) without evidence of hypersensibility
S3+: return of function (S3) with some return of two-point discrimination
S4: normal function
Sensory recovery classification on two-point discrimination alone:
Normal: less than 6 mm
Fair: 6 to 10 mm
Poor: 11 to 15 mm
IMAGING AND OTHER DIAGNOSTIC STUDIES
Diagnosis in acute injuries is usually based on history and clinical examination alone without need for additional investigations.
Plain radiographs are of little use in evaluation of the nerves themselves but may be helpful in cases of injury from fracture or projectiles.
Computed tomography (CT) myelography is useful for evaluation of injuries to the brachial plexus. The formation of a pseudomeningocele is indicative of root avulsion.
Magnetic resonance imaging (MRI) is useful for evaluation of peripheral injury but is not routinely indicated for peripheral nerve injuries.
Short tau inversion recovery (STIR) MRI may show enhancement of the nerve near the site of injury or interruption of the nerve trunk on T1- and T2-weighted images.
MRI provides visualization of pseudomeningoceles at the spinal cord levels in root avulsion injuries.
Electrodiagnostic testing
Nerve conduction velocity (NCV) and electromyography (EMG) are useful in evaluation of closed nerve injuries, for example, after fracture or multiple nerve injuries such as brachial plexus injury.
If stimulation distal to the suspected injury elicits a motor response about 3 days after injury, then the lesion is likely a conduction block. However, muscle action may be present in the case of complete transection for up to 9 days.
Fibrillation potentials on EMG appear after 2 to 3 weeks and indicate muscle denervation and a severe grade nerve injury.
Recovery is best evaluated with serial examination of compound muscle action potentials. Early recovery of only a few motor units may indicate reinnervation from adjacent intact nerves and should not be used as an indicator of recovery of the repaired nerve.
DIFFERENTIAL DIAGNOSIS
Muscle or tendon injury in open lacerations
Parsonage-Turner syndrome (brachial plexus neuritis)
Peripheral nerve entrapment
Partial nerve injury (neuroma in continuity)
Neurapraxic injury (axonal dysfunction without discontinuity)
NONOPERATIVE MANAGEMENT
Nonoperative management of a completely transected nerve after an open injury is doomed to failure because cut ends retract and scar tissue forms in the gap.
Pending recovery of the nerve, splinting of the paralyzed joint maintains functional position and range-of-motion exercises prevent contractures.
Serial clinical examination and electrodiagnostic testing are helpful to evaluate recovery.
SURGICAL MANAGEMENT
Nerves that have been completely interrupted require surgical measures to restore continuity.
All open injuries with neurologic impairment must be explored expeditiously.
With closed injuries or delayed presentation, consider the overall functional capacity of the injured limb.
In a largely motor nerve, for example, the radial nerve, tendon transfers may restore function more reliably than nerve repair.
Preoperative Planning
The cause of the peripheral nerve injury must be identified. The repaired nerve must have a favorable local environment if the repair is to be successful.
Underlying fractures must be stabilized.
Adequate soft tissue coverage of the nerve repair must be planned.
Repair should be delayed when multiple débridements are necessary until the bed for the repaired nerve is optimal and wound can be primarily closed. If delayed repair is considered, the distal and proximal ends should be identified and tagged with a suture in the epineurium.
Intraoperative nerve stimulation of motor or mixed (motor and sensory) nerves may assist with identification of the proximal and distal nerve stumps within the first 72 hours after injury. During this interval, residual neurotransmitters are present in the distal nerve and stimulation is still possible.
If segmental loss is suspected, as with a crushing injury, the patient must give informed consent for additional options such as conduit repair or nerve grafting.
Injuries that present late should be evaluated with electrophysiologic studies to look for signs of recovery.
If intraoperative nerve stimulation is to be used, muscle relaxants should be avoided at induction of general anesthesia.
If associated muscle or tendon lacerations are present, muscle relaxation facilitates their repair.
Regional anesthetics such as supraclavicular block provide excellent muscle relaxation, and a supraclavicular catheter will help in administering postoperative analgesia. However, the motor blockade from the block will prevent the use of intraoperative nerve stimulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree