Baseball players place significant stress across their shoulders and elbows during the throwing motion, causing unique patterns of injuries in the overhead throwing athlete. Specific nerve injuries include suprascapular neuropathy, quadrilateral space syndrome, and cubital tunnel syndrome. Nonoperative treatment includes cessation of throwing and symptom management. As symptoms improve, athletes should start rehabilitation, focusing on restoring shoulder and trunk flexibility and strength. The final rehabilitation phase involves an interval throwing program with attention directed at proper mechanics, with the goal of returning the athlete to competitive throwing. Surgery may assist in a positive outcome in particular patients who fail to improve with nonoperative treatment. Additional indications for surgery may include more profound neuropathy and nerve compression by a mass lesion.
Baseball is a sport requiring speed, coordination, strength and timing that is enjoyed by millions of people around the world. From a biomechanical perspective, the throwing motion, and in particular the pitching motion, places extreme stress on the throwing arm. Baseball pitchers are required to throw frequently, complicating the trauma placed across the pitcher’s throwing arm. This combination of high stress placed on the shoulder and elbow during the throwing motion, coupled with the cumulative microtrauma caused from repetitive overuse, places baseball pitchers at increased risk for peripheral nerve injuries in the upper extremity. Neuropathies of the shoulder comprise less than 2% of all causes of pain and weakness for patients in this region. Because of its uncommon nature, it is not unusual for the diagnosis of a neuropathy of the shoulder to be delayed. Signs and symptoms of a neuropathy typically overlap with more common causes of shoulder pain or weakness, further complicating diagnosis. Affected baseball players often have coexisting shoulder diagnoses such as rotator cuff pathology, labral tears, instability, and internal impingement, which may be the more dominant clinical finding. For example, patients who have suprascapular neuropathy may present with a more dominant impingement syndrome caused by to the weakness in the rotator cuff muscles as a result of the peripheral nerve injury. Baseball players are susceptible to nerve injuries seen in other sports (cervical radiculopathy, lumbar radiculopathy, “burners” or “stinger,” carpal tunnel syndrome). The focus of this article is on reviewing forms of peripheral neuropathies more common to baseball players and resulting from their unique throwing motion.
Biomechanics
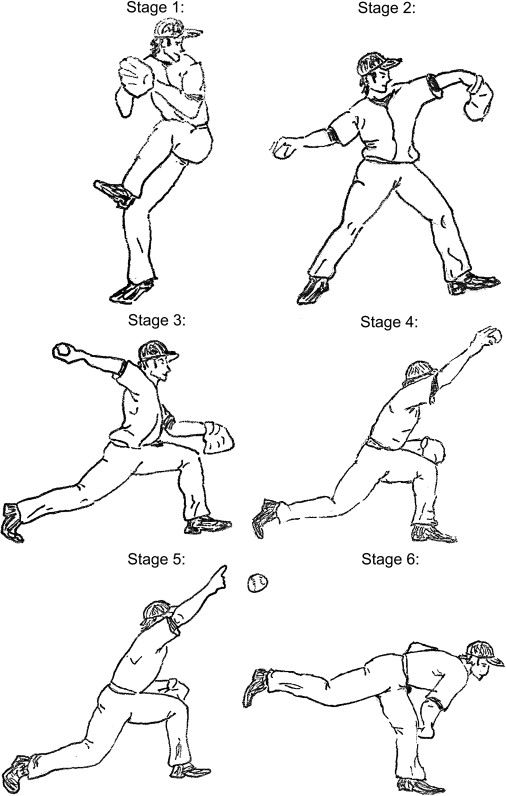
The overhand throwing motion in baseball, initially described as occurring in three phases, has subsequently been subdivided to occur in six distinct phases ( Fig. 1 ). The initial phase of the throwing motion, stage 1, is the windup. The windup involves individualize body mechanics; however, it begins with the stride foot coiling backward, away from home plate, and the arms swinging overhead, imparting little stress to the upper extremity. The next portion of the throwing motion is broken up into the early cocking phase (stage 2) and the late cocking phase (stage 3). The early cocking phase begins when the ball leaves the nondominant gloved hand and ends when the forward foot comes in contact with the ground. The late cocking phase begins with the planting of the striding leg and ends with the shoulder in a position of maximum external rotation, which can be as much as 170° to 180°. In the terminal portion of the late cocking phase, the torso begins to open up and a shear force of 400 N is generated across the anterior shoulder, with the firing of the rotator cuff muscles generating a compressive force of 650 N. During this phase the elbow is flexed between 90° and 120°, with forearm pronation increasing to 90°. Stage 4, the acceleration phase, rotates the shoulder internally at velocities of 7000 deg/sec, and ends with ball release. The acceleration phase occurs rapidly over a time period of approximately 0.05 seconds. During this phase the elbow accelerates as much as 3000 degrees/sec, with tremendous valgus stresses generated about the medial aspect of the elbow estimated as high as 64 N·m. The anterior bundle of the ulnar collateral ligament (UCL) complex bears the principal portion of these forces, with the secondary supporting structures being the flexor-pronator musculature. Most elbow injuries occur during this acceleration phase of throwing. Stage 5, the deceleration phase, begins with ball release and ends with the cessation of humeral rotation. It is during the deceleration phase that the energy generated through the first four phases is dissipated and the upper extremity decelerates at a rate of almost 6180 deg/sec over a time span of only 50 msec. During the deceleration phase a violent contraction of all muscle groups occurs, with eccentric contraction of the muscles required to slow down arm rotation. Stage 6, the follow-through, is the final stage of the overhead throwing motion. This is the rebalancing phase where the body moves forward with the arm until motion stops. The entire throwing motion takes less than 2 seconds.
Pathophysiology
Peripheral nerves are highly susceptible to injury from stretch and compression. Both mechanisms of injury result in nerve ischemia, edema, microenvironmental changes, and conduction impairment. These changes are proportional to the magnitude and duration of the insult, and can lead to irreversible damage. In a rabbit tibial nerve model, a stretch that increased in situ length as little as 6% resulted in conduction abnormalities. In this same model, a stretch of 15% leads to irreversible neural functional deficits. Intraneural vascular supply to a rabbit tibial nerve was compromised when the nerve was elongated by 8%, with total occlusion at 15%. Similar changes have been reported to occur with compression. The unique stresses and arm positions occurring during the baseball throwing motion likely place the peripheral nerves of the thrower’s dominant upper extremity at risk for similar and significant mechanical insults. If these nerve injuries are recognized early, cessation of throwing and an appropriate rehabilitation program may be of benefit and preventative of more permanent injury.
Pathophysiology
Peripheral nerves are highly susceptible to injury from stretch and compression. Both mechanisms of injury result in nerve ischemia, edema, microenvironmental changes, and conduction impairment. These changes are proportional to the magnitude and duration of the insult, and can lead to irreversible damage. In a rabbit tibial nerve model, a stretch that increased in situ length as little as 6% resulted in conduction abnormalities. In this same model, a stretch of 15% leads to irreversible neural functional deficits. Intraneural vascular supply to a rabbit tibial nerve was compromised when the nerve was elongated by 8%, with total occlusion at 15%. Similar changes have been reported to occur with compression. The unique stresses and arm positions occurring during the baseball throwing motion likely place the peripheral nerves of the thrower’s dominant upper extremity at risk for similar and significant mechanical insults. If these nerve injuries are recognized early, cessation of throwing and an appropriate rehabilitation program may be of benefit and preventative of more permanent injury.
Suprascapular neuropathy
Anatomy
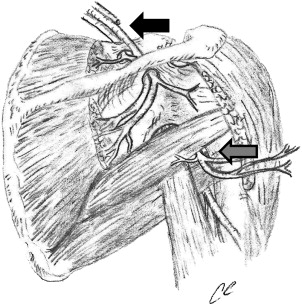
The suprascapular nerve is a mixed motor and sensory nerve that arises from the upper trunk of the brachial plexus (C5 and C6 nerve roots) and supplies motor innervations to the supraspinatus and infraspinatus muscles, and sensory innervations to the coracohumeral and coracoacromial ligaments, subacromial bursa, and acromioclavicular and glenohumeral joint ( Fig. 2 ). Although most anatomic studies have not identified any cutaneous innervation, cutaneous innervations of the proximal-lateral arm has been reported.
After the suprascapular nerve originates from the brachial plexus, it initially courses through the posterior triangle of the neck before traveling deep to the trapezius muscle, underneath the clavicle, toward the suprascapular notch. The suprascapular nerve next crosses the suprascapular notch, passing beneath the transverse scapular ligament, with the suprascapular artery and vein traveling above the ligament. After the suprascapular nerve traverses the suprascapular notch, it typically sends two motor branches to the supraspinatus muscle, as well as sensory branches to the coracoclavicular and coracohumeral ligaments, the acromioclavicular joint, and the subacromial bursa. After passing through the suprascapular notch, the main branch of the suprascapular nerve travels inferiorly to the infraspinatus muscle, while providing a sensory branch to the posterior aspect of the glenohumeral joint. Before reaching the infraspinatus muscle, the suprascapular nerve courses sharply around the spine of the scapular through a fibro-osseous tunnel formed by the spinoglenoid ligament and the spine of the scapula. The prevalence of the spinoglenoid ligament has been debated in the literature; however, more recent anatomic studies have identified the ligament in the majority of shoulder specimens examined. Finally, the suprascapular nerve terminates in two to four branches innervating the infraspinatus muscle.
Etiology
The suprascapular nerve may be injured by direct trauma, including surgery, traction, clavicle fractures or dislocations. Baseball players, however, typically sustain injury to the suprascapular nerve because of the frequent and significant stresses place on their arms during the throwing motion. Various hypotheses have been proposed to explain suprascapular nerve injuries, including traction, compression, and vascular compromise; however, there is no consensus on which cause, if any, is dominant. A common location of injury is where the suprascapular nerve passes through the suprascapular notch underneath the superior transverse scapular ligament. Injury at this location results in denervation, affecting both supraspinatus and infraspinatus muscles. Typically patients who have a suprascapular nerve injury at this location will present with posterior shoulder pain in addition to shoulder weakness.
Overhead throwing athletes seem to be particularly susceptible to distal injury to the suprascapular nerve at the spinoglenoid notch. Patients who have injury to the suprascapular nerve at the spinoglenoid notch often present with isolated infraspinatus muscle atrophy. Because this location of nerve injury is distal to the sensory fibers from the suprascapular nerve, there may be no history of shoulder pain The incidence of distal suprascapular nerve injuries in overhead throwing athletes is likely significantly higher than in the general population. Not limited to baseball, asymptomatic atrophy of the infraspinatus muscle was identified in 12 of 96 top-level volleyball players Similar findings were observed in major league professional baseball players, with isolated infraspinatus muscle atrophy present in 4% of major league pitchers. Infraspinatus muscle atrophy was more common among starting pitchers, more experienced pitchers, and pitchers who had thrown for more innings at the major league level, so amount of throwing appears to play a significant role.
An additional cause of suprascapular nerve injury is compression by a ganglion cyst. Baseball players can develop superior and posterior labral tears as a result of the stresses placed on the shoulder during the throwing motion. Ganglion cysts may develop because of labral tear acting as a one-way valve, permitting joint fluid to escape into the cyst cavity but not allowing the return of the synovial fluid back into the joint. The most common location for labral tears is the superior posterior labrum, which is probably why the most common location for a shoulder ganglion cyst is the spinoglenoid notch. This location of a ganglion cyst can result in compression to the suprascapular nerve once the ganglion cyst has reached sufficient size, leading to isolated denervation of the infraspinatus muscle.
Clinical Evaluation
History and examination
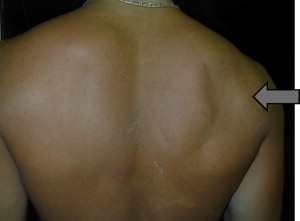
Patient presentation of suprascapular nerve injuries varies depending on the location of the nerve injury and the presence of additional shoulder pathology. Patients who have proximal lesions affecting the supra and infraspinatus muscles often present with posterior shoulder pain, atrophy of the involved muscles, and weakness of shoulder abduction and external rotation ( Fig. 3 ). A similar presentation can be seen in patients who have chronic rotator cuff tears; however, patients who have large rotator cuff tears are typically older than the overhead throwing athlete who has a suprascapular nerve injury. Patients who have a more distal suprascapular nerve injury are often asymptomatic, and the nerve injury presentation may result from casual observation of the scapular muscle atrophy only. A small percentage of patients who have a distal suprascapular nerve injury may have posterior shoulder pain, however. This pain may be caused by dysfunction in the relationship of the posterior capsules sensory branch to the spinoglenoid ligament. Finally, it is common for a patient’s initial presentation to be the result of other shoulder pathology. An example is patients who have a suprascapular nerve injury caused by a ganglion cyst, in which the labral tear associated with the ganglion cyst causes the shoulder pain leading to clinical attention.
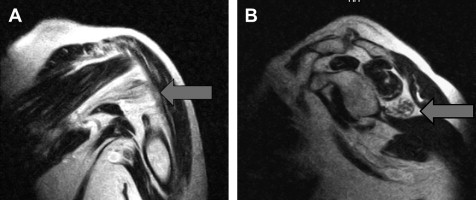
Diagnostic studies
Patients who have a suspected suprascapular nerve injury should be evaluated with MRI, which not only aids in the diagnosis of suprascapular nerve injuries but also helps in the differentiation of proximal nerve injuries from more distal nerve injuries. In one investigation, Kullmer and colleagues performed experimental denervation of the supraspinatus and infraspinatus muscles in rats by segmental excision of the suprascapular nerve. Identifiable muscle atrophy and increased signal intensity on T2-weighted MRI images appeared within 3 weeks of injury. MRI has the added advantage of being able to detect intra-articular lesions and ganglion cysts ( Fig. 4 ). Increased sensitivity and specificity for the diagnosis of labral tears can be obtained with the addition of intra-articular gadolinium contrast. MRI arthrography has been found to have a sensitivity of 91% and specificity of 93% for the diagnosis of labral lesions.
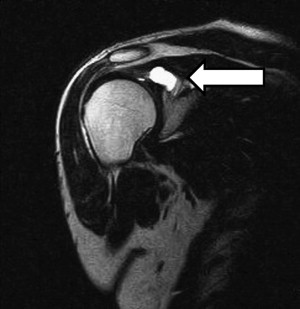
Electrodiagnositic studies are also important in confirming the diagnosis of a suprascapular nerve injury, localizing the site of the nerve injury, and by excluding other etiologies in the differential diagnosis, such as cervical radiculopathy and brachial plexopathy. An increased latency when performing the suprascapular compound motor action potentials (CMAP) indicates demyelination or a conduction block. Although there are sensory innervations from the suprascapular nerve, there are no cutaneous nerve branches capable of generating a sensory nerve action potential (SNAP). Electromyography (EMG) is also a useful method for evaluating axonal loss from a suprascapular nerve injury by the demonstration of increased involuntary spontaneous activity with fibrillations and positive sharp waves. These changes tend to occur 2 to 3 weeks after the injury. Disadvantages of these electrodiagnositic studies include their invasiveness and user dependence; however, EMG has a high sensitivity and specificity for diagnosing the suprascapular neuropathy, and may have prognostic value. For example, identification of polyphasic potentials with large-amplitude and long-duration motor unit action potentials (MUAPS) with disappearance of involuntary activity indicates collateral sprouting, reinnervation, and the absence of ongoing denervation.
Treatment
Nonoperative
Nonoperative treatment of suprascapular nerve injuries should be considered as the initial treatment option, unless nerve compression is caused by a mass lesion. Nonoperative treatment includes the avoidance of activities felt to be causative, obviously including cessation of throwing. This treatment should be combined with a rehabilitation program. The initial focus of a rehabilitation program is on shoulder flexibility, with particular attention given to improving internal rotation contractures, postural training, and strengthening of the periscapular and deltoid muscles. Strengthening of the rotator cuff muscles can be started once symptoms of nerve impingement have subsided. The advancement of the rehabilitation program should be monitored closely to avoid reinjury to the nerve while it is healing. In general, success rates with nonoperative treatment are less satisfactory when the nerve injury results from a mass lesion such as a ganglion cyst. One concern with nonoperative treatment is that a delay in decompression of the suprascapular nerve may result in incomplete restoration of muscle function. It has been demonstrated that fatty atrophy in the rotator cuff muscle is not reversible following repair of large rotator cuff tears. A similar situation likely occurs when portions of rotator cuff muscles (supraspinatus and infraspinatus) are denervated following nerve injury.
Operative treatment
The operative treatment of suprascapular nerve injuries depends on the etiology and location of the nerve lesion. When planning the surgical procedure it is important to understand the nerve injury location and the presence or absence of a ganglion cyst.
Surgical treatment of a suprascapular nerve injury when not related to a ganglion cyst is generally through a posterior surgical incision. When the nerve injury is located at the suprascapular notch, surgical management involves resection of the superior transverse scapular ligament, with or without deepening the suprascapular notch. When the nerve injury is located at the spinoglenoid notch, surgical decompression involves the release of the spinoglenoid ligament, which is present in the majority of patients. Additionally, some authors advocate deepening the spine of the scapula; however, if the spine of the scapular is deepened more than 1 cm, the acromion is at risk of fracture. Surgical decompression, whether performed for a proximal or distal nerve injury, typically leads to resolution of patient’s pain and a substantial increase in muscle strength; however, the return of muscle bulk is less predictable and requires many months to occur. Reports of arthroscopic decompression of the suprascapular nerve have been described recently, but these techniques remain new and investigational.
A different scenario exists when a ganglion cyst is compressing the suprascapular nerve; a shoulder arthroscopy should be performed to assess for a coexisting labral tear. The ganglion cyst can be drained using arthroscopic techniques at the site of the labral tear, following which the labral tear should be repaired. Ganglion cysts can also be excised through an open incision; however, shoulder arthroscopy should be performed in this scenerio to address any underlying labral pathology It is possible for ganglion cyst to recur following an open cyst excision when a labral tear has not been addressed. In one case report of a failed excision of a ganglion cyst, at a subsequent operation, arthroscopy identified a labral tear, which after correction led to no further recurrence of the ganglion cyst.
Axillary neuropathy
Anatomy
The axillary nerve supplies motor function to the deltoid and teres minor muscles, as well as providing sensation over the lateral shoulder. The axillary nerve is the terminal branch of the posterior cord of the brachial plexus, which arises from the C5 and C6 nerve roots. The nerve travels along the subscapularis muscle obliquely, and then courses under the axillary recess of the glenohumeral joint. The nerve subsequently exits posteriorly, accompanied by the posterior humeral circumflex artery, passing through the quadrilateral space, which is bound by the teres minor superiorly, the teres major inferiorly, the long head of the triceps medially, and the humeral shaft laterally (see Fig. 2 ). The axillary nerve divides into two major trunks after exiting the quadrilateral space. The posterior trunk provides motor innervations to the posterior deltoid muscle and the teres minor muscle before terminating as the superior lateral brachial cutaneous nerve supplying sensation to the lateral aspect of the shoulder. The anterior truck of the axillary continues anteriorly to the undersurface of the deltoid muscle, coursing around the humeral shaft to supply the middle and anterior deltoid muscle.
Etiology
Axillary nerve injuries are uncommon and represent less than 1% of all nerve injuries. Injury to the axillary nerve in baseball players can occur as a result of a direct trauma or as the result of quadrilateral space syndrome. Direct trauma to the nerve can result from a traction injury, as can be seen with shoulder dislocations or fractures. The risk of injury to the nerve with shoulder dislocations increases with patient’s age, severity of the trauma, and with the length of time that a shoulder is left unreduced. Additionally, a direct blow to the outer aspect of the shoulder can injure the axillary nerve by compressing it against the proximal humerus. Another etiology for axillary nerve injuries is the quadrilateral space syndrome, an uncommonly reported syndrome identified in overhead throwing athletes in which the axillary nerve is compressed within the quadrilateral space. The most frequent reported cause of quadrilateral space syndrome is a fibrous band within the quadrilateral space. The nerve may also be compressed because of a space-occupying lesion (ie, ganglion cyst). Repetitive microtrauma may also play a causative role. Intermittent compression of the axillary nerve occurs when the shoulder is a position abduction and external rotation. This shoulder position occurs during the late cocking phase of pitching, and results in closing of the quadrilateral space by contraction of teres minor and teres major.
Clinical Evaluation
History and physical examination
Patients who have an axillary nerve injury following a direct trauma to the shoulder often provide a history of the shoulder trauma followed by decreased sensation over the lateral aspect of their upper arm. Patients may also report a history of weakness or fatigue of the arm with overhead activity or heavy lifting. Patients who have quadrilateral space syndrome generally do not report a history of trauma. It is not uncommon for the diagnosis to be delayed, and the condition may only be identified after failed treatment for other shoulder diagnoses. Pain is described as a dull ache or burning located over the posterior aspect of the shoulder. Asymmetry of the deltoid muscle or “squaring off” of the affected shoulder may be noted as a result of the deltoid atrophy. On physical examination, patients typically have tenderness over the region of the quadrilateral space, which may lessen with a diagnostic injection of local anesthetic. Although a positive FABER test (shoulder forward elevation, abduction, and external rotation held for more than 1 minute) may be suggestive of this diagnosis by leading to shoulder pain, this nonspecific finding may also occur in more common shoulder diagnoses such as internal impingement or impingement syndrome. Shoulder strength tests should be performed because the teres minor accounts for 45% of external rotation strength and the deltoid muscle provides the majority of strength in shoulder flexion, abduction, and extension. If the posterior deltoid muscle and teres minor muscles are not affected, then an axillary nerve injury may have occurred distal to the quadrilateral space. During the history and physical examination it is important to evaluate for other more common neurologic etiologies within the differential diagnosis, including cervical pathology, thoracic outlet syndrome, or brachial plexus pathology. Additionally, clinical evaluation should also assess for intrinsic shoulder pathology such as adhesive capsulitis, osteoarthritis, and rotator cuff tears.
Diagnostic studies
Plain radiographs are important imaging studies to obtain, particularly when there is a history of trauma. In acute trauma, an EMG/nerve conduction velocity (NCV) should be obtained 3 weeks after the shoulder injury in order to confirm the diagnosis and aid in evaluating the severity of the nerve injury. As with assessment of the suprascapular nerve, EMG evaluation of other muscles will assist in ruling out a nerve root, brachial plexus, or other peripheral nerve injury. Like the suprascapular nerve, CMAP is obtainable and there is no readily obtainable SNAP. In patients who have quadrilateral space syndrome, EMG/NCV is not as sensitive, and may not become useful until later in the disease process. MRI will often be helpful in evaluating these patients ( Fig. 5 ) through detection of changes of denervation in affected muscles as well as detection of ganglion cysts or other mass lesions compressing the axillary nerve. Additionally, MRI aids in evaluating for other causes of shoulder pain and dysfunction. It is not uncommon for the diagnosis of quadrilateral space syndrome to be suggested by an MRI performed to examine for other causes. In a prospective investigation, isolated teres minor atrophy was identified in 12 out of 217 patients (5.5%) who underwent consecutive shoulder MRI examinations over a 3-month period of time. Subclavian arteriography can evaluate for compression of the axillary nerve by an arterial structure, such as with the posterior humeral circumflex artery as it passes through the quadrilateral space. Typically, the angiogram does not show reduced blood flow through the posterior humeral circumflex artery when the patients arm is held in adduction. With abduction and external rotation of the shoulder, however, the blood flow in the artery is attenuated. A concern with this test is the low specificity for making the diagnosis of quadrilateral space syndrome. One investigation using MR angiography demonstrated that 80% of asymptomatic patients had occlusion of arterial flow in the posterior humeral circumflex artery when the arm was placed in abduction and external rotation.