Chapter 76 Partial Denervation for the Treatment of Painful Neuromas Complicating Total Knee Replacement
For a patient to perceive pain, there must be a neural pathway for transmission of the impulses generated from injured tissues. This concept is easily accepted for the skin, where a direct injury may disrupt, crush, or stretch a nerve that innervates a cutaneous distribution. Although the mechanisms to be discussed were introduced for upper extremity pain in the 1980s, it was not until the description of the human knee joint in 199426 that the application to knee pain could be developed.15,16
An analogy is helpful to provide the theoretical framework for the lower extremity application. If after an injury to the dorsoradial aspect of the distal forearm, a patient complained of pain in that region, and physical and radiographic evaluation suggested that the musculoskeletal system was without abnormalities, a neuroma or compression of the radial sensory nerve would be the likely source of pain. If anatomic variations of that region of skin were considered carefully, the differential diagnosis would be expanded to consider an injury to the lateral antebrachial cutaneous nerve, which overlaps with the radial sensory nerve in 75% of people.38 Similarly, if a ganglion were removed from the dorsal aspect of the wrist, and the postoperative incision was painful and remained painful, this would raise the possibility of a neuroma of a branch of these same two cutaneous nerves. However, if the patient’s pain after ganglion removal were deeper and became worse with wrist flexion and extension, a nerve pathway that involved the dorsal wrist capsule would have to be considered as the cause of the pain. The terminal branch of the posterior interosseous nerve innervates the dorsal wrist capsule and in particular the scapholunate ligament.17 A neuroma of this nerve may have resulted when the ganglion was excised from a portion of this ligament. If the patient’s pain did not resolve with therapy, corticosteroid massage, or injection, and if the pain limited hand function, successful treatment could be achieved by appropriate treatment of the involved nerve. To identify the involved nerve, nerve blocks would be used to identify the source of pain as one or both cutaneous nerves, or the nerve that innervates the wrist joint, or all three nerves. For a cutaneous neuroma, treatment must include resection of the neuroma, or at least interruption of its neural pathway, because the neuroma can be a source of pain.44 For the dorsoradial aspect of the hand, the appropriate treatment is to transfer the proximal end of the cutaneous nerve into a proximal large muscle, such as the brachioradialis.39 For the dorsal wrist, a partial (dorsal) wrist denervation is indicated.6
Pathophysiology of Neuroma Formation
In the peripheral nervous system, the response to division of a peripheral nerve has been well documented. This response involves degeneration of the distal axons, survival of the distal Schwann cells, sprouting of the proximal nerve fibers (because this is the physiologic response of the cell bodies located in the dorsal root ganglion), and production of nerve growth factors by the distal (denervated) Schwann cells. This response results in regeneration of the proximal axon sprouts distally toward the chemotactic gradient of nerve growth factors. These sprouts track along the basement, where fibronectin, type I collagen, and laminin also attract the sprouts.7,40 No biologic tool is currently available to prevent this cascade of events. Without destruction of the dorsal root ganglion, neuroma formation always occurs.
Treatment of a Painful Neuroma
A painful neuroma can be managed successfully if the following three steps are followed:
Studies in monkeys have shown that when the proximal nerve sprouts into an environment of innervated muscle, and when that muscle is chosen to have minimal excursion, classic end-bulb neuromas do not form.42 Clinically, this approach has proven successful for the upper and lower extremities—the radial sensory and lateral antebrachial cutaneous nerves into the brachioradialis muscle,39 the palmar cutaneous branch of the median nerve into the pronator quadratus muscle,20 the plantar digital nerves into the foot intrinsic muscles,8 and the superficial and deep peroneal nerves into the anterolateral compartment muscles.9 For neuromas related to nerves that innervate joints, resection of the neuroma, or the peripheral nerve in an area proximal to the joint, usually allows the resected proximal end of the nerve to lie in an internervous plane, and direct muscle implantation is not needed. Examples are the anterior and posterior interosseous nerves in the forearm.6,7,13
History of Partial Joint Denervation in the Extremities
In 1966, Wilhelm53 published in German his approach to the treatment of persistent wrist joint pain. This concept became available to non–German-reading surgeons in 1977, when Buck-Gramcko4 reviewed the German-speaking experience with 313 patients. With an 80% follow-up that exceeded 2 years, their findings included excellent results in 69% of 195 patients. This was a complex review that included results from many surgeons, with 10 separate nerves being resected circumferentially about the wrist during a total wrist denervation. Many patients had “incomplete” or partial denervation done based on local anesthetic blocks. The patient population included patients who had persistent pain after forearm or wrist fracture, advanced arthritis, carpal instability, or severe sprain. Poorest results were seen in patients with unstable wrists, who later required fusions attempts.
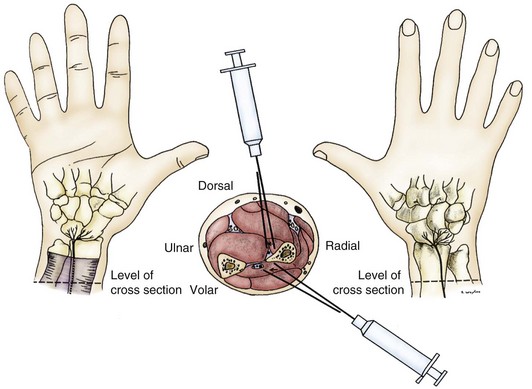
In 1978, Dellon and Seif17 published a description of the innervation of the dorsal wrist capsule by the terminal branch of the posterior interosseous nerve. This nerve could be identified easily over the distal dorsal forearm, proximal to the wrist. This publication implied that if wrist pain, regardless of its cause, could be isolated to neural transmission along this nerve pathway, that pathway alone could be interrupted, eliminating wrist pain—thus, this was a partial, rather than a total, approach to wrist denervation. This hypothesis was tested in 29 patients studied between July 1981 and June 1984.6 Pain in these patients was caused by wrist fracture (n = 8), carpal instability (n = 4), arthritis (n = 3), severe wrist sprain (n = 10), or dorsal ganglionectomy (n = 4). Range of wrist motion and grip strength were measured, an anesthetic block of the posterior interosseous nerve was performed, and range of motion and grip strength were then reassessed (Fig. 76-1). For the patient to be considered a surgical candidate, pain relief, increased range of motion, and increased grip strength had to be present. Results indicated that 90% of patients achieved pain relief and improved wrist function, and 83% returned to work. Failure occurred in three of the four patients with carpal instability, who required a subsequent wrist fusion attempt. Three patients had reflex sympathetic dystrophy after their wrist fracture; two of these patients had previously undergone Darrach procedures, and two had had a carpal tunnel decompression. Two patients with reflex sympathetic dystrophy had subjective improvement after partial dorsal wrist denervation and showed improved range of motion, but none of the three returned to work. Investigators concluded that partial joint denervation was effective in relieving pain and improving function in selected patients, that total joint denervation was not necessary, and that structural instability was a contraindication to partial joint denervation.
Logically, a group of patients could be identified for whom anterior wrist pain could be treated by a partial volar wrist denervation procedure. In 1984, Dellon and colleagues,13 after basic anatomic dissections to identify the terminal branch of the anterior interosseous nerve distal to the pronator quadratus muscle, attempted partial volar wrist denervation in a small group of patients. From April 1982 through March 1984, 11 patients with persistent volar wrist pain were identified; 9 had work-related injuries and 2 were involved in motor vehicle accidents. Each patient had good relief of pain, increased wrist range of motion, and increased grip strength after a block of the anterior interosseous nerve (see Fig. 76-1). At a mean follow-up of 12.8 months (range, 4 to 24 months), all patients had good to excellent relief of their pain. Four patients had returned to their regular job, three returned to “light duty” jobs, and four were in vocational rehabilitation. Investigated concluded that partial joint denervation was effective in relieving pain and improving function in selected patients, and that total joint denervation was not necessary.
In 1993, Dellon and Horner10 reviewed the experience of 51 patients treated for wrist pain with partial denervation. Overall, a 98% improvement in pain was noted, with the visual analog scale level decreasing in all patients by between 6 and 8 points out of 10. Improved range of motion occurred in 60% of the whole group. No patients with carpal instability were included in this more recent group.
Cutaneous Innervation of the Knee Region
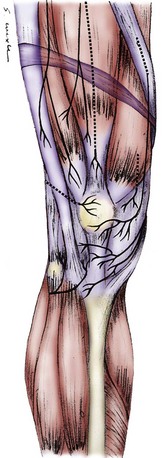
Virtually all anatomy books agree on the presence of the saphenous nerve innervating the infrapatellar region. This nerve originates medially from the femoral nerve and descends into the medial thigh through the adductor canal to emerge around or through the sartorius muscle. The infrapatellar branch divides from the femoral nerve variably: in the proximal third of the thigh (17.6%), in the middle third of the thigh (58.8%), and in the distal third of the thigh (23.5%).26 In 86% of people, two saphenous nerve branches are present in Hunter’s canal.26 In this situation, the more anterior of the two branches becomes the infrapatellar branch of the saphenous nerve, which when it enters the medial knee region is about 4 mm wide and begins to branch as it approaches the anterior midline. It always crosses the leg below the patella and usually has branches from below the patella to below the tibial tuberosity. The infrapatellar branch of the saphenous nerve innervates the anterior midline of the region below the patella and the lateral region below the patella. It does not innervate the skin covering the patella (Figs. 76-2 and 76-3). It may send branches into the distal anterior knee joint capsule.26
The skin covering the patella is innervated by the medial cutaneous nerve of the thigh, which is a better name for this nerve than the anterior or medial femoral cutaneous nerve because this nerve almost always branches from the saphenous nerve and travels as a separate branch, superficial to (39.1%), through (30.4%), or deep to (30.5%) the sartorius muscle, to emerge in the region of the femoral condyle, lying in the superficial subcutaneous plane.26 This nerve may range in size from 0.6 to 1.1 mm, and it may include more than one branch. Its branches often lie directly over the medial retinacular nerve, and a local anesthetic block in this region blocks both nerves (see Figs. 76-2 and 76-3).
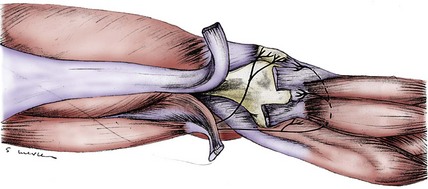
It is unclear whether the obturator nerve contributes branches to one or both of the branches of the saphenous nerve. The sensory branches of the obturator nerve enter into the complex of nerves within the adductor canal and do not emerge as a single identifiable branch in this distal thigh or knee region. Branches of the obturator nerve do participate in the innervation of the posterior knee capsule (Fig. 76-4).
The distal saphenous nerve theoretically begins distal to the infrapatellar branch of the saphenous nerve. Usually it has a separate branch in the thigh, or it may be the continuation of the saphenous nerve after the infrapatellar branch. Most often, regardless of its origin, the distal saphenous nerve gives off one or more transverse branches in the region distal to the tibial tuberosity; these may become a source of pain from long anterior or medial incisions. The terminal skin innervated by the distal saphenous nerve includes the medial dorsum of the foot and a region posterior to the medial malleolus (see Figs. 76-2 and 76-3).
The anterior femoral cutaneous nerve originates from the femoral nerve in the groin region and terminates at the patellar region. This nerve has many small (<1 mm) branches in this region and does not usually have an identifiable neuroma (see Fig. 76-2).
The lateral femoral cutaneous nerve begins at the hip region as a continuation of the L1 and L2 nerve roots. It has a branch that innervates the lateral buttock and a branch that innervates the lateral thigh skin extending to the level of the lateral knee. At the level of the inguinal ligament, the lateral femoral cutaneous nerve measures 3 to 7 mm. At the lateral knee region, it has terminal branches only, which usually are too small to identify. Although pain can be referred to the knee from compression of the lateral femoral cutaneous nerve at the hip, direct injury to the knee or surgery in the knee region almost never causes a neuroma of this nerve. Lateral knee skin pain is almost always due to a neuroma of the medial cutaneous nerve of the thigh or the infrapatellar branch of the saphenous nerve (see Fig. 76-2).
Innervation of the Knee Joint
The innervation of the human knee joint has been described by Ruedinger (1857),51 Druner (1927),18 Jeletsky (1931),30 Gardner (1948),24 Wilhelm (1958),54 Kennedy and coworkers (1982),33 and Wojtys and associates (1990).55 None of these descriptions were derived from a large number of fresh human cadavers, and none provided detailed anatomic drawings sufficient to reveal optimal surgical approaches to these nerves. Clarification of nomenclature was required. A study reported in 1994 by Horner and Dellon26 served as the basis for the following description and nomenclature. In that study, 45 fresh cadaveric adult knees were dissected using loupe magnification. The following description is based on that study and has been supplemented by clinical and operative experience over the past 15 years.
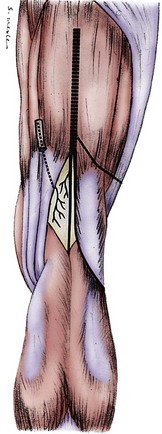
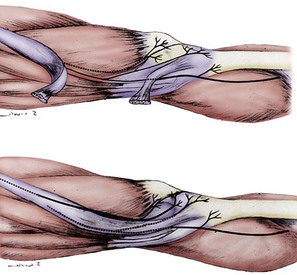
The lateral retinacular nerve is consistently present. It arises directly from the sciatic nerve, proximal to the popliteal fossa, and continues laterally to go beneath the biceps femoris tendon to reach the lateral retinacular structures. The lateral retinacular nerve lies beneath the lateral retinaculum. At this level, it exists as two or three 1-mm branches entering into the deeper structures of the knee joint. It is always accompanied by the recurrent lateral geniculate vessel and always is just distal to the vastus lateralis muscle. It is immediately superficial to the synovial structures of the knee joint (Fig. 76-5; see Figs. 76-2 and 76-4).
The medial retinacular nerve is consistently present. It arises from the branch of the femoral nerve that innervates the vastus medialis muscle. It exits from beneath the distal posterior aspect of the vastus medialis muscle and travels anteriorly to innervate the medial retinacular structures in 90% of subjects, whereas in the other 10%, it exits through the muscle to innervate the medial retinacular structures.26 The medial retinacular nerve lies beneath the medial retinaculum, and at this level exists as two or three 1-mm branches entering into the deeper structures of the knee joint. It is always accompanied by the recurrent medial geniculate vessels and always is just distal to the vastus medialis muscle. It is immediately superficial to the synovial structures of the knee joint (see Figs. 76-2 and 76-3).
Nerves to the prepatellar bursal structures arise from the terminal branches of femoral nerves that innervate the vastus intermedius muscle. These continue distal to the muscle, lying on the anteromedial side of the distal femur, to enter into the prepatellar bursa and its surrounding structures. No unique name has been given to these nerve branches, each of which is approximately 1 mm in diameter (see Fig. 76-2).
Nerves to the posterior knee capsule arise from the sciatic nerve over a 2-cm distance and widely innervate the posterior knee structures. No unique name has been given to these nerve branches, each of which measures less than 1 mm in diameter (see Fig. 76-4). Druner18 observed in 1927 that the posterior knee capsule received branches from the obturator nerve through Hunter’s canal. The confluence of these branches with the branches from the sciatic nerve was named the popliteal plexus by Ruedinger.51
Innervation of the Proximal Tibiofibular Joint
The common peroneal branch of the sciatic nerve provides branches that innervate the proximal tibiofibular joint. These branches do not have a unique name. One branch arises proximal to the fibular head, and one or two arise just distal to the fibular head. These branches are small, generally less than 1 mm in diameter, and require loupe magnification for identification. They travel into the structures between the fibular head and Gerdy’s tubercle of the tibia (see Figs. 76-2, 76-4, and 76-5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree