Parainfluenza Viruses
Caroline Breese Hall
Major actors of the yearly show that fills
Each fall with varied forms of respiratory ills
and striking melodies that mimic far too well
The barking seal, the crowing cock, the brassy bell.
— CBH
The parainfluenza viruses (PIVs) cause ubiquitous infections with varied manifestations in childhood. Their spectrum of illness ranges from mild and asymptomatic upper respiratory tract illness to disease involving the lower respiratory tract in young children. Recognition of the PIVs as important worldwide human pathogens comes not only from their causing symptomatic illness early in life, but also from their ability to reinfect throughout life. The PIVs, especially types 1 and 2, have earned a distinctive notoriety from their being the most frequent causes of croup. In 1991, PIV-1 and PIV-2 infections were estimated to account for 250,000 visits to emergency rooms, 70,000 hospitalizations, and $190 million in medical care costs annually.
The first PIV was isolated from mice in Sendai, Japan a half century ago and received the name Sendai virus, or the hemagglutinating virus of Japan (HVJ). The PIVs subsequently were recognized to be widespread in animals. The first human PIV was isolated from infants with croup and was descriptively named croup-associated (CA) virus. With the subsequent recognition of PIV-1, PIV-3, and PIV-4, CA virus was renamed PIV-2.
VIRUS CHARACTERIZATION
The PIVs, along with respiratory syncytial virus (RSV), belong to the family of enveloped RNA viruses, Paramyxoviridae, which have nonsegmented, single-stranded, negative-sense genomes. The genomes of all four types of human PIVs code for at least one nonstructural and six structural proteins. The lipid envelope, derived from the host cell, is studded with the surface glycoproteins, hemagglutinin-neuraminidase (HN) and fusion (F) proteins. These two surface glycoproteins are integral to the immunity and pathogenesis of the PIVs. The HN facilitates the attachment of the virus to the host cell, and the F protein, along with HN, allows the virus to enter the host cell, by fusing the host and viral cellular membranes. Cell-to-cell spread of infection subsequently occurs.
The human PIVs possess common antigens, as demonstrated by their serologic responses to heterotypic parainfluenza strains and to mumps virus. The human PIVs may be divided into two major antigenic groups (PIV-1 and PIV-3 in one group, and PIV-2 and PIV-4, along with mumps virus, in the other). Minor antigenic variation occurs with all four PIV types.
EPIDEMIOLOGY
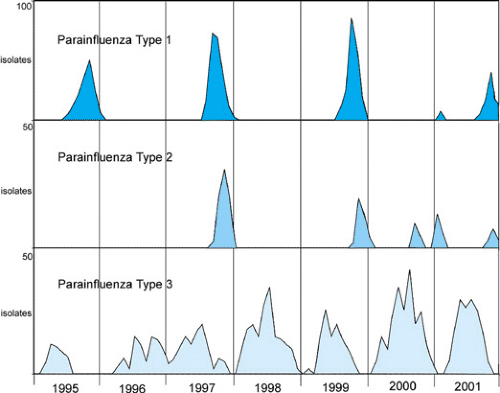
The PIVs have been found across the globe, and, although their seasonal pattern may vary according to the climate and geography, their clinical manifestations are similar. In the United States, the seasonal pattern of the PIVs in different parts of the country may have some variation, but PIV-1, -2, and -3 each has a character that is distinctive and yet interactive with the other serotypes (Table 189.1). The PIVs did not demonstrate their epidemic nature until the mid-1960s, when PIV-1 showed a notable pattern of outbreaks occurring every other year in the fall of odd-numbered years (Fig. 189.1). PIV-2 has a more unpredictable behavior than does PIV-1 or PIV-3 (Fig. 189.1). PIV-2 most often appears toward the end of the PIV-1 fall outbreak, but sporadically and with less intensity than does PIV-1. PIV-3 is present in the community for more months than are the other PIVs. It usually is most prominent in the spring to fall of each year. Relatively few isolations and outbreaks of PIV-4 have been reported, and disease associated with PIV-4 usually is mild. Thus, relatively little is known about the epidemiologic and clinical manifestations of PIV-4.
Most children have been infected with PIV-1, -2, and -3 by the time they reach 5 years of age, and many have been
reinfected by this age. PIV-3 usually is the child’s earliest infection, with one-half to two-thirds of infants acquiring infection within the first year of life. Infection with PIV-1 and PIV-2 usually occurs subsequently and more gradually, with approximately three-fourths of school-age children having antibody. Seroprevalence to PIV-2 is variable, ranging anywhere from 50% to 90%. Almost all adults have detectable antibody to PIV-1 and -3.
reinfected by this age. PIV-3 usually is the child’s earliest infection, with one-half to two-thirds of infants acquiring infection within the first year of life. Infection with PIV-1 and PIV-2 usually occurs subsequently and more gradually, with approximately three-fourths of school-age children having antibody. Seroprevalence to PIV-2 is variable, ranging anywhere from 50% to 90%. Almost all adults have detectable antibody to PIV-1 and -3.
TABLE 189.1. PARAINFLUENZA VIRUSES | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Repeated infections with the PIVs may occur at any age, but many (50% or more) are asymptomatic. Boys tend to be hospitalized more frequently, but infection rates in outpatients and in populations with repeated infections suggest that gender does not play a permanent role.
The importance of PIV-1, -2, and -3 in causing acute respiratory infections in children has been supported recently by data from the National Respiratory and Enteric Virus Surveillance System from the Centers for Disease Control and Prevention (CDC). Although the rate of hospitalization with PIV infection was widely variable in different years and locations, researchers estimated that rates of PIV-1, -2, and -3–associated hospitalizations ranged from 1.9 to 12 per 1,000 children younger than 1 year of age and between 0.5 and 2.0 per 1,000 children ages 1 to 4 years. These findings resulted in an estimated 7,600 to 48,000 hospitalizations for infants younger than 12 months of age, and 8,100 to 42,600 admissions for those 1 to 4 years of age. PIV-3 and PIV-1 were the most frequent causes of hospitalization, ranging from 8,700 to 52,000 annual hospitalizations for PIV-3 and 5,800 to 28,900 for PIV-1. PIV-2 generally was a less common cause, but with wide variation, causing 1,800 to 15,600 hospitalizations each year.
PATHOGENESIS
Transmission
The PIVs are highly contagious, as evidenced by the alacrity of spread among children in day-care centers and other groups of young children in close contact. Almost all children initially exposed to PIV-3 in these settings will acquire infection. Approximately 60% to 80% will acquire PIV-1 and PIV-2 under similar circumstances.
The mode of spread of the PIVs appears similar to that of RSV, thus requiring close person-to-person contact and direct exposure to the contaminated secretions of infected
individuals. This exposure most likely occurs primarily via the large-droplet aerosols of respiratory secretions from infected contacts or by contact with contaminated fomites followed by self-inoculation. The large quantities of virus contained in the nasal secretions of young children, the frequency of both symptomatic and asymptomatic infections, and PIVs’ ability to survive in the environment support the likelihood that these modes of transmission predominate. Experimentally, PIV-3 has been shown to be viable in a small-particle aerosol for 1 hour, but secretions most likely are disseminated via the coughs and sneezes that are so prominent in PIV infections. PIV-3 primarily is contained in large-particle aerosols for which close person-to-person contact is required.
individuals. This exposure most likely occurs primarily via the large-droplet aerosols of respiratory secretions from infected contacts or by contact with contaminated fomites followed by self-inoculation. The large quantities of virus contained in the nasal secretions of young children, the frequency of both symptomatic and asymptomatic infections, and PIVs’ ability to survive in the environment support the likelihood that these modes of transmission predominate. Experimentally, PIV-3 has been shown to be viable in a small-particle aerosol for 1 hour, but secretions most likely are disseminated via the coughs and sneezes that are so prominent in PIV infections. PIV-3 primarily is contained in large-particle aerosols for which close person-to-person contact is required.
Immunopathology
The inoculation of these viruses into the upper respiratory tract usually results in infection after an incubation period of 2 to 4 days. Attachment of the virus occurs via specific receptors on the cell membrane of the host’s respiratory epithelium, with subsequent penetration occurring by fusion. Infections may be limited to the upper respiratory tract or subsequently spread to the lower respiratory tract, which most frequently occurs with PIV-3. Infections with PIV-1 and -2 tend to involve primarily the larynx and upper trachea, resulting in croup.
Few pathologic studies of children with PIV infection exist. The spread of the viral infection along the respiratory tract results in inflammation of the epithelium, with the accumulation of necrotic tissue and inspissated mucus. This inflammatory material may cause obstruction in both the upper and lower respiratory tracts. In the former, this obstruction may result in the characteristic nasal congestion and otitis media. Spread to the lower respiratory tract results in obstruction of the flow of air, with atelectasis, pneumonia, and bronchiolitis developing in the young child. Involvement of the subglottic tissues may be the prominent manifestation, resulting in stridor and croup.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree