8
Pain: Its Pathogenesis and the Evolution of Chronic Pain
 Local and Radiating Pain
Local and Radiating Pain
Local and radiating pain due to intervertebral disk disease are generated by the irritation of different components of the nervous system. The pain may be referred, i.e., it may be felt in the distribution of an irritated segmental nerve. Referred pain, by definition, is not felt in the same location as the causative lesion. Brachialgia and sciatica due to nerve root irritation are two common types of referred pain.
Referred pain differs from organ pain that is felt at the site of the causative lesion, as in local cervical and lumbar syndromes. There are also certain pain states arising from dysfunction of the sympathetic nervous system, mainly in the area of the body supplied by the cervical nerve roots. The pain in these cases is generally constant, diffusely localized, and accompanied by vasomotor and trophic changes. The sympathetic origin of the pain is reflected in its fluctuation according to the circadian rhythm and other hormonal cycles, as well as its greater frequency in autonomically labile individuals.
Though many symptoms in the back evade precise definition, most disk-related complaints can be attributed in some way to the involvement of particular neural structures in the vicinity of the disks.
 Nerve Supply of the Spine
Nerve Supply of the Spine
Pain-sensitive structures. The human intervertebral disk contains no nerve fibers. Sensory nerve endings have been found to date only in the outermost layers of the anulus fibrosus underlying the posterior longitudinal ligament (Kuhlendahl 1950, Kuhlendahl and Richter 1952, Mulligan 1957, Mendel et al. 1992, Bogduk 2000, Fagan 2003).
These histological findings have their physiological counterpart in the experimental findings of Smith and Wright (1958), who, in the course of disk surgery, attached fine nylon fibers to various structures in the motion segment, as well as to the nerve root. Postoperatively, they were able to duplicate the patient’s typical symptoms only by pulling on the posterior portion of the anulus fibrosus, or on the nerve root itself. Kuslich and Ulstrom (1990) stimulated various tissues in the motion segment during lumbar disk operations under local anesthesia and recorded the sensitivity of each to pain. Pain was most easily produced by stimulation of the skin and the compressed nerve root, followed by the anulus fibrosus and the posterior longitudinal ligament. The ligamentous attachments and the joint capsules were less commonly pain-sensitive, while the ligamentum flavum, lumbar fascia, lamina, facet cartilage, and noncompressed nerve roots were completely insensitive to pain.
Puncturing the disk from its anterior aspect, as in cervical discography, is entirely painless. If the disk is intact, no pain or pressure sensations arise when contrast medium or dye is injected into it. On the other hand, if there is a disk protrusion making contact with a nerve root, expansion of the disk may stretch the nerve root, producing pain. This has diagnostic value (see the discussion of the distension test in Chapter 9).
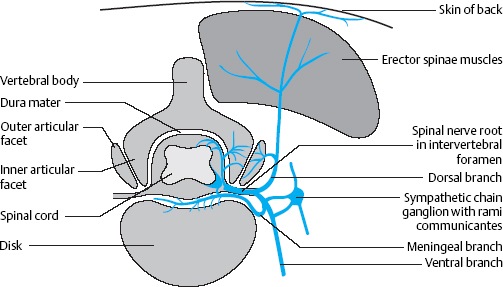
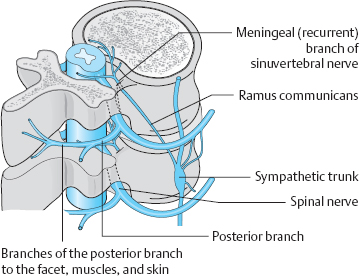
Fig. 8.1 “Autoinnervation” of the structures of the motion segment by the meningeal branch of the spinal nerve. Sensory fibers originate mainly in the intervertebral joint, the posterior longitudinal ligament, and the spinal nerve itself.
Medial transdural disk puncture at lumbar levels produces no more than a transient, lumbago-like pain at the precise moment that the posterior longitudinal ligament and the dorsal anulus fibrosus are punctured. Just as in disk prolapse surgery under local anesthesia, pressure on the dorsal edge of the disk with a probe induces typical, severe low back pain.
The pain-sensitive structures in the spinal canal— mainly the posterior longitudinal ligament and the dorsal anulus fibrosus, but also the periosteum and intervertebral joint capsules—are supplied by a special spinal nerve. In 1850, von Luschka described a branch of the spinal nerve distal to the spinal ganglion, the so-called meningeal branch, which receives sympathetic fibers from the sympathetic chain and then re-enters the spinal canal through the intervertebral foramen (von Luschka 1850). This branch is also called the recurrent branch or the sinuvertebral nerve. Von Luschka’s observations were later confirmed by Hovelacque (1925), Roofe (1940), Wiberg (1949), and Bogduk (2000).
The meningeal branch of the spinal nerve branches further after its re-entry into the spinal canal to provide an efferent, afferent, and sympathetic nerve supply to the inner portions of the intervertebral joint capsule, the vertebral periosteum, the posterior longitudinal ligament, and the spinal meninges.
 Mechanically irritable sensory nerve endings are found primarily in the posterior longitudinal ligament, the intervertebral joint capsule, and the spinal nerve itself.
Mechanically irritable sensory nerve endings are found primarily in the posterior longitudinal ligament, the intervertebral joint capsule, and the spinal nerve itself.
The mixed spinal nerve contains motor, sensory, and sympathetic fibers. As it exits the spinal canal by way of the intervertebral foramen, it splits into a ventral and a dorsal branch. The much larger ventral branch supplies the anterior region of the body, including the limbs, while smaller dorsal branch supplies the skin and muscles of the back. Further branches supply the outer facets of the intervertebral joints and their capsules. The paired dorsal branches, from the occiput to the coccyx, pierce the paravertebral fascia to supply the posterior portions of the corresponding dermatomes. In the thoracic region (T1–T11), the portion of the dermatome supplied by the dorsal branch is relatively narrow, i.e., close to the midline. In the lumbar area, it extends laterally to the outer edge of the erector spinae muscles, and, in the sacral area, it extends to the dorsal foramina. Richter (1977) described entrapment neuropathies of these nerve branches as being responsible for certain painful syndromes, particularly occipital neuralgia and coccygodynia.
The pattern of nerve supply suggests certain mechanisms for the generation of pain in the motion segment and implies that painful disk syndromes are likely to be complex (Fig. 8.1).
 The Foramino-articular Region
The Foramino-articular Region
The foramino-articular region of the lower lumbar segments is the site of exit of the spinal nerve from the intervertebral foramen and of its division into terminal branches (see Chapter 11). Its anatomy is, therefore, of special clinical interest, particularly with respect to therapeutic approaches involving local injections.
Figures 8.1–8.3 show the close proximity of the spinal nerves and their branches, their connections to the sympathetic trunk by way of the rami communicantes, and the muscles and joints in this region. The nociceptors of the joint capsules, the posterior longitudinal ligament, and the vertebral periosteum lie directly adjacent to afferent fibers of various nerves. Structural and functional disturbances, mostly caused by the early disk degeneration that is characteristic in humans, lead to reactive changes in the joint capsules. A further consequence (both direct and indirect) is the autonomic response that is induced by irritation of the ramus communicans from the meningeal branch to the sympathetic trunk and mediated by the spinal nociceptive reflex arc. The presence of so many pain-sensitive structures within a compact space suggests the possibility of effective treatment of pain through local injections of anesthetics or anti-inflammatories.
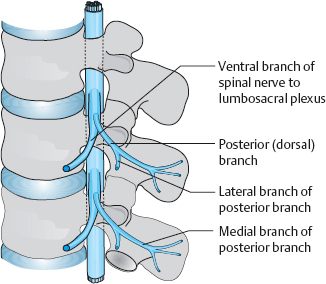
Fig. 8.2 Lumbar spinal nerve with ventral and dorsal branches (from Krämer and Nentwig, after Bogduk 1997).
 Discogenic Pain
Discogenic Pain
Aside from purely mechanical causes of pain, there are other types of stimuli that might irritate the pain-sensitive nerve endings of the motion segment. Just as in other organs (e.g., the teeth or stomach), changes of osmolarity, pH, or chemical composition must be considered as possible causes of motion segment pain.
Nachemson (1969) measured the pH in prolapsed intervertebral disks in vivo with an antimony needle electrode. pH values below 7 were associated with certain inflammatory reactions at the nerve roots that were not seen with pH values in the alkaline range. When the pH was 6.1 or lower, the nerve roots were encased in dense, reactive scar tissue. The chemical composition of the prolapsed disk tissue, independently of its size, can also partially account for the severity of the patient’s symptoms. Even in the absence of disk prolapse, nerve structures near the disk (e.g., in the posterior longitudinal ligament) can be irritated by chemical changes taking place within it. Just as in other organs, the poor metabolic condition of the disk sets chemical processes in motion that alter the osmolarity and pH of disk tissue. Nachemson (1969) hypothesized that proteolytic enzymes in connective tissue lysosomes catalyze the release of acidic amino acids from the protein–polysaccharide complexes of the disk matrix, thereby lowering the pH. It may be additionally lowered by an increased concentration of free acidic mucopolysaccharides. Karlsson et al. (1968) and Diamant et al. (1968) reported an inverse relationship between the intraoperatively measured intradiscal pH and lactate levels: when the pH was low the lactate level was high, and vice versa.
The anatomical and biochemical findings of MacCarron et al. (1987), Mooney (1987), and Fagan (2003) suggest that purely discogenic pain—i.e., pain arising in the disk itself—is possible. Thus, if the hydrostatic pressure on a disk is kept high, dissolved acidic metabolites may be pressed out of the disk and excite an inflammatory response in neighboring nerve fibers. If there is a tear in the anulus fibrosus, not much compressive pressure on the disk will be needed for this to take place.
Further studies on discogenic pain have been published by Schwarzer et al. (1995), Nakamura et al. (1996), Coppes et al. (1997), Faustmann (2004), Kniesel (2004), Schaaf et al. (2004), Weisskopf et al. (2004), Peng et al. (2005), and Murata et al (2006a, 2006b).
 Spinal Deformities without Pain
Spinal Deformities without Pain
 The severity of pain in the motion segment is correlated less closely with the size of the structural abnormality than with the period of time over which it arose.
The severity of pain in the motion segment is correlated less closely with the size of the structural abnormality than with the period of time over which it arose.
Even very marked deviations of the spinal axis, with torsion, spondylosis, and osteochondrosis, may be asymptomatic if they have developed over many years. The nerve roots, ligaments, and intervertebral joint capsules can apparently adapt adequately to such changes in the long term. On the other hand, even a small dorsal disk protrusion can produce severe pain if it arises suddenly and makes (even minimal) contact with pressure-sensitive neural elements of the posterior longitudinal ligament or the nerve root.
 Pain Arising from the Posterior Longitudinal Ligament
Pain Arising from the Posterior Longitudinal Ligament
Pain arising from the posterior longitudinal ligament is dull and poorly localizable. It may be of sudden and very intense onset, as in acute lumbago or acute neckache. It may also develop gradually, as when the dorsal aspect of the intervertebral disk is placed under abnormally high tension for long periods of time by pronounced kyphosis or by an abnormal increase of disk volume. The meningeal branch of the spinal nerve is responsible for intrinsic spinal pain. It is currently unclear whether compression of the spinal dura mater causes pain. Evidence against this possibility comes from the fact that median disk prolapses and spinal tumors sometimes produce little or no pain.
 Radicular Pain
Radicular Pain
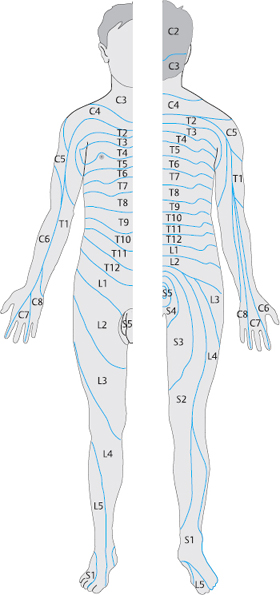
Mechanical nerve root irritation. Among all structures of the peripheral nervous system, the spinal nerve root is by far the most vulnerable to injury. Compression and stretching are the most common mechanisms. Pain is generated only by pressure on, or pulling of, the meningeal (and thus mesenchymal) tissue that encases the preganglionic segment of the dorsal root. Figure 8.4 shows the segmental distribution of radicular pain by dermatomes.
Radicular pain has the following general features:
 The pain radiates along the dermatome.
The pain radiates along the dermatome.
 Associated muscle atrophy does not fit the pattern of distribution of any peripheral nerve.
Associated muscle atrophy does not fit the pattern of distribution of any peripheral nerve.
 Any reflex deficit that is seen is attributable to a radicular rather than peripheral nerve lesion.
Any reflex deficit that is seen is attributable to a radicular rather than peripheral nerve lesion.
 There is no accompanying autonomic nerve deficit.
There is no accompanying autonomic nerve deficit.
Spinal nerves contain fibers supplying the skin and muscle of the trunk and limbs, as well as the spine itself. Thus, nerve root lesions can produce various combinations of symptoms. The segmental distribution of symptoms is most easily recognizable when the structures innervated by the ventral branch of the spinal nerve are prominently involved. The diagnostician should remember, however, that nerve root lesions produce abnormalities not only in the area supplied by the ventral branch on the anterior trunk and limbs, but also in the muscles supplied by the dorsal branch.
Compression of a spinal nerve root produces clearly demarcated segmental sensory and motor disturbances on the trunk and in the limbs. The precise location and severity of the nerve root lesion will determine whether the most prominent symptoms reflect involvement of the ventral or dorsal nerve branch. Dermatomal pain may be of different kinds, depending on the type and severity of nerve root compression; the symptoms may range from isolated, referred pain (band-like pain), without any “objectifiable” signs, to total anesthesia. Smith and Wright (1958) showed that the distance the pain radiates is proportional to the pressure on the nerve root. For example, if a lumbar nerve root is only lightly compressed, sciatica radiates only to the thigh, but stronger compression makes it radiate into the foot.
Cervical and lumbar nerve root compression generally produce no autonomic deficit. Mumenthaler et al. (1998) emphasize the fact that no autonomic efferent fibers to the sympathetic chain exit the spinal cord above T2 or below L2. Thus, the segments most commonly affected by acute and chronic disk disease, C5–C8 and L4–S1, lie well outside the portion of the spinal cord containing the main sympathetic nucleus (the intermediolateral cell column). The absence of accompanying autonomic nerve symptoms, e.g., sweating, in cervical and lumbar radiculopathy is an important diagnostic clue by which these processes can be distinguished from peripheral nerve lesions.
The degree to which a nerve root is compressed by a bulging disk depends both on changes in the volume and consistency of the disk tissue and on position-related changes in the motion segment. Many of the diagnostic and therapeutic measures that are undertaken in patients with disk-related complaints rely on this simple fact.
In previous chapters, we have pointed out that the intervertebral disks of an adolescent or young adult are not mechanically stiff elements, but rather self-contained connective tissue organs whose shape and volume normally fluctuate in response to mechanical stress, relaxation, and asymmetrical compression. The other components of the motion segment, including the nerve fibers, must adapt to these fluctuations. The normally existing space between the posterior border of the disk and the dural sac, with its paired exiting nerve roots, enables movements of the spine, changes of the disk contour, and changes of nerve root length to take place without putting the nerve root under excessive tension or compression. This “reserve space” between the disk and the dura mater contains fat and epidural veins; its width varies among segments. In the intervertebral foramen, too, there is normally enough space between the nerve root and the bony canal in which it is enclosed. The nerve root may be compressed, however, when either of these reserve spaces is narrowed by a disk protrusion or prolapse, or by osteophytes, enlarged vessels, or bony overgrowth (spinal stenosis).
 If an intervertebral disk makes contact with a spinal nerve, the physiological, pressure-dependent fluctuations in the consistency and volume of the disk are transmitted to the nerve. The nerve may not have room to move as freely and may therefore be a source of pain when movement of the spine exceeds a certain range in a given direction.
If an intervertebral disk makes contact with a spinal nerve, the physiological, pressure-dependent fluctuations in the consistency and volume of the disk are transmitted to the nerve. The nerve may not have room to move as freely and may therefore be a source of pain when movement of the spine exceeds a certain range in a given direction.
This explains the highly variable character of disk-related complaints. A nerve root may be compressed not only by disk tissue, but also by bony structures. Though the most common cause is a cervical osteophyte arising from the uncinate process, nerve roots can be compressed in the lumbar spine, too, by arthrotic extensions of the intervertebral joints or spondylotic osteophytes on the posterior surface of the vertebrae. The pain of osteogenic nerve root compressiontypically fails to resolve spontaneously or to respond to conservative treatment. It is usually sharply localized, because the same part of the nerve is always irritated. Surgical treatment is often necessary.
When prolapsed disk tissue enters the epidural space through an anular tear and comes into direct contact with the nerve root, there can also be a biochemically induced irritation of the nerve root. Disk tissue normally is not found in the spinal canal, and, when it is deposited there, it excites a foreign-body reaction. Saal and Saal (1989), Olmarker and Rydevik (1993), Willburger et al. (1998), and Olmarker (2004) have experimentally demonstrated the direct toxic effect of disk material on nervous tissue.
 The pain of disk prolapse is caused not just by mechanical trauma, but also by chemical irritation of the nerve root.
The pain of disk prolapse is caused not just by mechanical trauma, but also by chemical irritation of the nerve root.
Mechanical and chemical irritation produce grossly visible changes in the nerve root: initially edema, later atrophy. At surgery, the nerve root is sometimes seen to have a reddish or bluish-purple discoloration. The compressed nerve fibers within the root fire action potentials spontaneously. Compression causes demyelination of nerve root axons and alters their excitability (Wehling 1993, Olmarker 2004, Gupta et al. 2005).
 An already inflamed nerve root is more than normally sensitive to additional mechanical irritation.
An already inflamed nerve root is more than normally sensitive to additional mechanical irritation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree