Cold Paediatrics
Mr Andreas Rehm
Paediatric Orthopaedic Surgeon
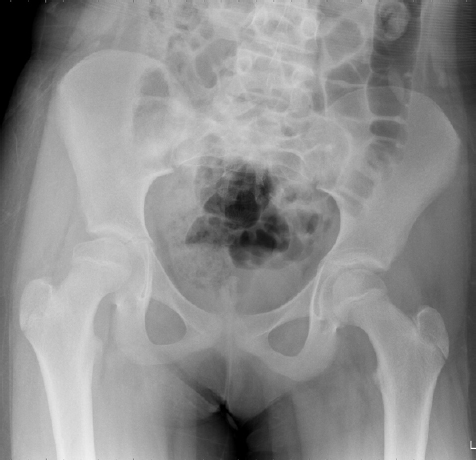
1. What are the X ray findings?
-Deformity of the right femoral head
-Subchondral fracture with collapse of the articular surface
-Lateral subluxation of the femoral head
-Break in Shenton’s line
-leg is held in adduction
2. What is Perthes Disease?
It is an idiopathic avascular necrosis of the femoral capital epiphysis. The cause is unknown. It is thought to be multifactorial.
3. Epidemiology
It occurs between about 18 months of age and skeletal maturity.
Most children are between 5 and 10 years of age at the time of onset.
It is bilateral in 10 to 12% of children. Boys are affected 4 to 5 times more frequently than girls. Girls have a worse prognosis.
4. Etiology
The main arterial supply to the femoral head comes from the lateral ascending cervical artery which is the terminal branch of the medial circumflex femoral artery and lies within the hip joint capsule. The terminal branch runs through a narrow passage between the greater trochanter and the capsule where it can become constricted. The blood supply can be occluded by abducting and internally rotating the hip.
Inoue et al concluded from their research that at least two femoral head infarcts are required to cause Legg-Calvé-Perthes disease.
Inoue A, Freeman MA, Vernon-Roberts B. The pathogenesis of Perthes disease. J Bone Joint Surg Br 1976; 58:453.
5. Most common symptoms and signs?
Symptoms are pain in the groin, around the hip and/or knee. Signs are:
-child walks with a limp
-limited range of hip movements affecting generally abduction and internal rotation.
6. What is the lateral Pillar classification (Herring)?
Group A
No involvement of the lateral pillar. No density change. No loss of height.
Group B
Mild density change in lateral pillar. Height ≥ 50% of original height. Central pillar collapse.
Group B/C
Very narrow lateral pillar (2-3 mm wide) with 50% of the original height that is depressed relative to the central pillar.
Group C
Lateral pillar with <50% of original height.
It has superseded the Catterall classification and has become the most commonly used classification.
Park et al evaluated the inter- and intra-rater reliability of the Herring lateral pillar, Catterall and Salter-Thompson classification and concluded that the Herring classification showed the greatest reliability.
Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calve-Perthes disease. J Pediatr Orthop 1992;12:143-150.
Herring JA. Tachdjian’s Pediatric Orthopaedics, Vol. 3, Fourth ed. Philadelphia: Saunders Elsevier, 2008:2519.
Park MS, Chung CY, Lee KM, Kim TW, Sung KH. Reliability and stability of three common classifications for Legg-Calvé-Perthes disease. Clin Orthop Relat Res 2012 Mar 20 (Epub ahead of print).
7. What is the Catterall classification?
Group I: | Only anterior portion of the epiphysis affected. Up to 25% head involvement. |
Group II: | More of the anterior portion is involved and a central segment is present. Up to 50% head involvement. |
Group III: | Most of the epiphysis involved with the unaffected portions located medial and lateral to the central segment. Up to 75% head involvement. |
Group IV: | Total head involvement. |
According to Catterall, groups I and II have a benign prognosis.
Several interobserver studies have shown a low degree of reproducibility.
Christensen F, Soballe K, Ejsted R. The Catterall classification of Perthes’ disease: An assessment of reliability. J Bone Joint Surg Br 1986; 68:614.
Hardcastle PH, Ross R, Hamalainen M. Catterall grouping of Perthes’ disease: An assessment of observer error and prognosis using the Catterall classification. J Bone Joint Surg Br 1980;62:428.
8. What are Catterall’s Head at risk signs?
1) Gage sign (V shape lucency at lateral epiphysis)
2) Horizontal growth plate
3) Lateral calcification
4) Subluxation
5) Metaphyseal cystic changes
Forster et al reported poor inter- and intra-observer reliability for head at risk signs.
Forster MC, Kumar S, Rajan RA, Atherton WG, Asirvatham R, Thava VR. Head-at-risk signs in Legg-Calvé-Perthes disease: poor inter-and intra-observer reliability. Acta Orthop 2006;77(3):413-417.
9. What are the 4 radiographic stages described by Waldenström?
1) initial / ischaemic
2) fragmentation/resorbtion
3) reossification/healing
4) residual/remodelling
Waldenström H. The definite form of coxa plana. Acta Radiol 1922;1:384.
Initial phase: The blood supply of the femoral head is compromised. The articular cartilage still grows as it is nourished by the joint fluid resulting in increased joint space and apparent mild joint subluxation on plain radiographs (Waldenstrom’s sign). The head ceases to enlarge. An increased density in the femoral head is the result of new bone accumulation on the dead trabeculae. At the end of this phase lucencies occur within the ossific nucleus and cysts within the metaphysis. Plain radiographs may show a subchondral fracture.
Fragmentation phase: More new bone is laid down on the dead trabeculae causing increased bone density. Subchondral fractures may occur causing a black subchondral line (Crescent sign). The hyperaemia and revascularisation causes bone lysis and rarefication giving a fragmented appearance on the plain radiographs.
Reossification phase: New subchondral bone is laid down in the femoral head until the entire head has re-ossified. The head is plastic and if it is not concentrically contained within the acetabulum, it will become deformed.
Residual phase: There are no further changes in the density of the femoral head. The shape of the femoral head may remodel. The shape of the acetabulum and greater trochanter may be affected. The plasticity is lost and the femoral head shape will remain. The normal internal architecture will return, but within an altered shape if this has occurred. Deformity will lead to arthritis.
10. Treatment
This is very controversial with there being no national and no international agreement. Annamalai et al (2007) showed a great deal of variability in the UK in the decision-making process and treatment.
In the early onset group under the age of 8 years, children are mostly managed non-operatively.
Herring et al conducted the largest multicentre centre study in America by comparing non-operative management with operative management (either femoral varus or pelvic osteotomy) for early and late onset groups. It was reported that there was no difference in outcome between non-operative management, femoral varus or pelvic osteotomy in the early onset group.
In the late onset group, they found an improved outcome for lateral pillar groups B and B/C with either femoral varus or pelvic osteotomy over the non-operative group. There was no difference for groups A and C.
Herring JA, Kim HT, Browne R. Legg-Calvé-Perthes Disease. Part I: Classification of radiographs with the use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am 2004 ;86-A:2103-2120.
Herring JA, Kim HT, Browne R. Legg-Calvé-Perthes Disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am 2004. 86-A:2121-2134.
Containment surgery has been advocated by others when the femoral head extrudes from the acetabulum irrespective of age (the femoral head is maintained within the depth of the acetabulum with femoral osteotomy, pelvic osteotomy or both combined).
Staheli and Catterral have popularised the shelf procedure to create a pelvic shelf providing resistance to further subluxation of the femoral head. Abduction bracing/casting can be used, but is generally not longer used by most paediatric orthopaedic surgeons.
In the late healed stage a femoral valgus osteotomy is used if there is hinge abduction. Combination of femoral and pelvic osteotomy have been described to improve the acetabular roof alignment in the healed stage.
Hip distraction using a hip joint spanning external fixator has been described for the late onset group to unload the femoral head by distracting the hip joint.
Stulberg classification describes the end stage of the disease at skeletal maturity. I: normal spherical femoral head; II: round femoral head and fitting within 2 mm of a circle on both anteroposterior and lateral radiograph; III: out of round by more than 2 mm on either radiograph; IV: flat head and matching flat acetabulum (aspherical congruency); V: flat head with non-matching acetabulum (aspherical incongruency).
11. Prognosis
This is related to the congruency of the hip joint and the spericity of the femoral head.
Patients with Stulberg class V develop severe arthritis before the age of 50 years.
StulbergSD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am 1981;63:1095.
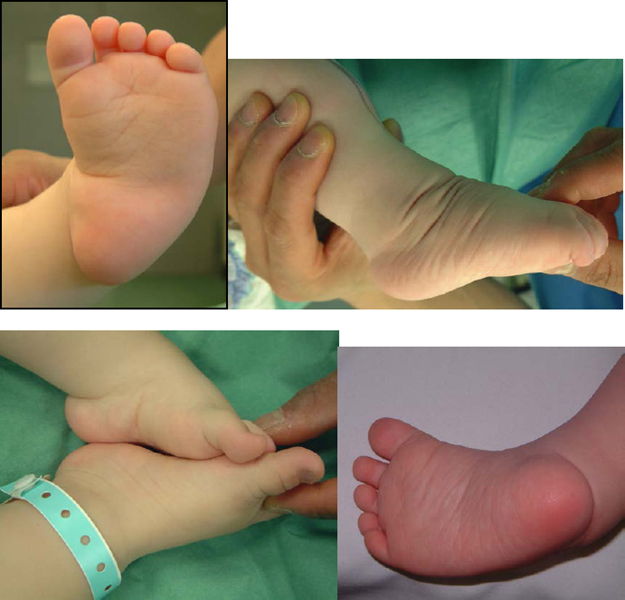
2. Congenital Taliped Equino Varus (CTEV)
Which are the deformities?
Deformities
1. Hindfoot equinus
2. Hindfoot varus
3. Midfoot/forefoot cavus
4. Forefoot adduction
CAVE = CavusAdductusVarusEquinus
The forefoot looks supinated but it is in a pronated position in relation to the midfoot. The calf and foot are smaller which is obvious in the unilateral deformity.
What is the Pirani classification.
This is a system to score the severity of a clubfoot deformity. It consists of A) a hindfoot score, assessing the posterior heel/ankle crease, the position of the calcaneum in the heel and the rigidity of the equinus and B) a midfoot score, assessing the medial crease, the lateral curvature of the foot and the lateral coverage of the head of the talus by the navicular.
Each component scores 0, 0.5 or 1 giving a maximum of 6 points for the most severe deformity.
Posterior heel/ankle crease: 0=normal (multiple fine creases which do not change the contour of the heel); 0.5=one or two deep creases which do not appreciably change the contour of the heel; 1=one or two deep creases which appreciably change the contour of the heel.
Position of the calcaneum in the heel: 0=calcaneum easily palpable; 0.5=calcaneum palpable deep inside the heel; 1=not palpable.
Rigidity of equinus: 0=foot comes up to a dorsiflexed position of more than 5°; 0.5=range between 5° of plantar flexion and 5° of dorsi flexion; 1=fixed equinus of more than 5°.
Medial crease: 0=normal (multiple fine creases which do not change the contour of the arch); 0.5= one or two deep creases which do not appreciably change the contour of the arch; 1= one or two deep creases which appreciably change the contour of the arch.
Curvature of lateral foot border: 0=straight lateral border from the heel to the 5th metatarsal head; 0.5=mildly curved lateral border (the curvature appears to be in the distal part of the foot in the area of the metatarsals; 1=pronounced curvature (it appears to be at the level of the calcaneo-cuboid joint).
Lateral talar head coverage: 0=complete reduction of the navicular onto the talar head; 0.5=partial reduction of the navicular onto the talar head; 1=easily palpable talar head because of fixed medial subluxation of navicular.
Pirani S, Outerbridge HK, Sawatzky B, Stothers K. A reliable method of clinically evaluating a virgin clubfoot evaluation. 21st SICOT Congress 1999.
How will you manage- at birth and late stage.
The Ponseti method is the preferred treatment. It starts soon after birth and consists of:
1. Weekly serial casting with above knee plasters for about 6
weeks.
2. Percutaneous Achilles tendon release in about 80% of
patients at about 6 weeks.
3. Further post-operative casting for about 3 weeks (a cast
change during this period might be necessary).
4. Boots on a bar 23 hours a day for 3 months.
5. Boots on a bar for during the night up to the age of 4/5 years.
Ponseti treatment is also effective in older children in correcting all or part of the deformity. Depending on the severity of the deformity additional surgery is required.
Ponsetti method
1. Simultaneous correction of cavus, fore/midfoot adduction (aim for 60°-70° abduction) and hindfoot varus.
2. Equinus correction once cavus, fore/midfoot adduction and hindfoot varus are corrected.
About 20% of patients need a tibialis anterior tendon transfer for dynamic supination deformity between the age of 3 to 5 years. Transfer is into the lateral cuneiform (the ossification centre must be visible).
Outcome of ponseti?
Boden et al reported a significant reduction in the need for radical surgical release with the Ponseti technique in comparison to a stretch and strap technique. Gray et al performed a metaanalysis of the literature to evaluate interventions for clubfeet. Evidence was limited because of limited use of outcome measures and lack of available raw data. From the data available they concluded that the Ponseti technique may produce better short-term outcomes compared with the Kite technique. Jowett et al performed a systematic review of the literature of the results of the Ponseti method and concluded that the original Ponseti method is the current best practice for the treatment of clubfeet with an initial correction rate of around 90%. Halanski et al performed a prospective comparative study comparing the Ponseti method with below knee casting followed by surgical release. They concluded that both had a relatively high recurrence rate but that the Ponseti cohort had significantly less operative interventions and required less revision surgery. Therefore they adopted the Ponseti method as their primary treatment for clubfeet.
Morcuende et al reported that 86% of 157 clubfoot patients treated with the Ponseti method underwent a percutaneous Achilles tendon release. The need for surgical releases was avoided in 98% of their cohort.
Boden RA, Nuttall GH, Paton RW. A 14-year longitudinal comparison study of two treatment methods in clubfoot: Ponseti versus traditional. Acta Orthop Belg 2011. 77(4):522-8.
Gray K, Pacey V, Gibbons P, Little D, Frost C, Burns J. Interventions for congenital talipes equinovarus (clubfoot). Cochrane Database Syst Rev 2012.18;4.
Jowett CR, Morcuende JA, Ramachandran M. Management of congenital talipes equinovarus using the Ponseti method: a systematic review. J Bone Joint Surg Br 2011. 93(9):1160-4.
Halanski MA, Davison JE, Huang JC, Walker CG, Walsh SJ, Crawford HA. Ponseti method compared with surgical treatment of clubfoot: a prospective comparison. J Bone Joint Surg Am 2010. 92(2):270-8.
Morcuende JA, Dolan LA, Dietz FR, Ponseti. Radical reduction in the rate of extensive corrective surgery for clubfoot using the Ponseti method. Pediatrics 2004. 113(2):376-80.
A minority of patients will need extensive soft tissue releases. The majority of these are thought to be the result of non-compliance with the Ponseti treatment by parents/carers. Teratologic clubfeet generally also need surgical releases. Residual deformities might require osteotomies and/or fusions at a later stage. This is preferably delayed until the end of growth (girls ~14 years, boys ~16 years).
Etiology
The etiology is unknown. It is thought to be multifactorial with genetic and extrinsic factors being involved. The incidence is about 1-2 (0.39 – 7) in 1000 but there are racial differences. There is a ~4% chance of a child to have a clubfoot deformity if one parent had a clubfoot deformity. There is a ~15% chance of a child to have a clubfoot deformity if both parents had a clubfoot deformity.
Pathology
The talus is the primary defect.
Thickening and contracture of tendon sheaths and ligaments, denervation and neuromyogenic changes of muscles, shortened musculotendinous units, fibrosis of tissues and deficiencies of arteries have been described.
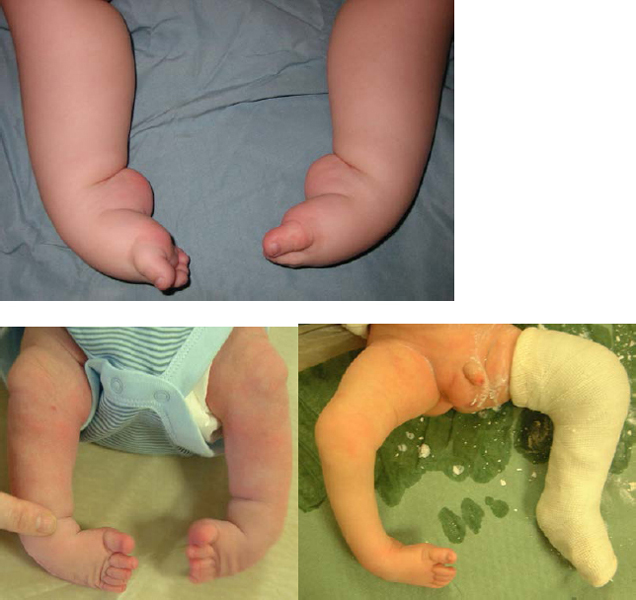
3. Developmental Dysplasia of the Hip (DDH)
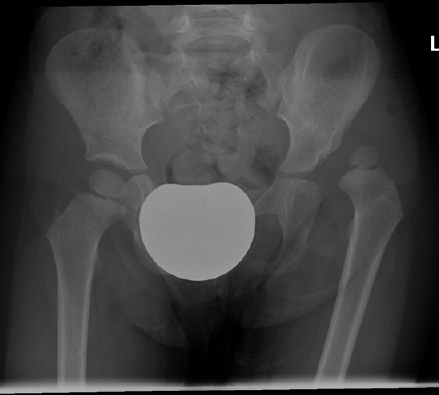
Dislocated hip in 3 years old girl.
What would be the clinical findings?
1) Apparent shortening and external rotation of the leg.
2) Reduced abduction of the affected hip.
3) There might be asymmetry of the thigh and/or buttock skin creases (dislocated hips can have symmetric looking creases and many normal hips have asymmetric creases).
4) The femoral head is most likely palpable in the buttock.
5) Barlow and Ortolani test are most likely negative since dislocated hips in older patients are usually irreducible. It is most likely possible to feel the femoral head moving within the buttock.
Describe Barlow and Ortolani test
Barlow test (described 1962, T.G. Barlow, Manchester): The hips are flexed to 90°. The thigh is held between index finger and thumb with the index finger on the greater trochanter and the thumb on the medial side of the thigh. The middle-, ring- and little fingers are at the back of the hip joint. The leg is adducted towards the midline whilst pressure is applied on the knee, directing the force posteriorly. If the hip is dislocatable you will feel the femoral head coming out into the buttock (positive test).
Ortolani test (described 1937, M. Ortolani, Italian Paediatrician): From the above position the leg is abducted slowly and the greater trochanter is pushed anteriorly. A positive sign is an obvious clunking sensation when the femoral head reduces into the acetabulum.
Which lines and angles do you assess on the antero-posterior X ray?
2. Hilgenreiner line (German surgeon and orthopaedist), Prague, 1870-1954): This is a straight line drawn through the upper aspect of both triradiate cartilages.
3. Perkins line (Orthopaedic Surgeon, Oxford, 1892-1979): Drawn perpendicular to Hilgenreiner’s line through the supero-lateral aspect of the acetabulum.
Perkins and Hilgenreiner line divide the hip joint into 4 quadrants. The proximal medial femur or the ossification centre of the femoral head lies within the lower medial quadrant in a normal hip.
4. Acetabular index: This is the angle between Hilgenreiner’s line and a line drawn from the triradiate cartilage to the lateral edge of the acetabulum. It should measure less than 20° by the age of 2 years.
What is the normal Graf ultrasound alpha angle?
Graf type I = normal = ≥60 degrees
Graf type
IIa = alpha angle 50-59°, up to the age of 3 months
IIb= alpha angle 50-59°, over the age of 3 months
IIc=alpha angle 43-49°, beta angle <77°
IId =alpha angle 43-49°, beta angle >77°
Graf type III =alpha angle <43°, everted labrum.
Graf type IV =alpha angle <43°, the femoral head is dislocated with the labrum interposed between femoral head and acetabulum (inverted labrum).
Treatment
1) 0-6 months of age
All babies have their hips examined clinically after birth using Barlow and Ortolani test. Most hips with a positive Barlow test at birth stabilise within 2 to 3 weeks without treatment. An ultrasound (US) should be done to confirm the clinical diagnosis. These hips need to be followed up with ultrasounds to assure normal development. Treatment with a Pavlik harness is indicated if the hips do not stabilise after 2 to 3 weeks. If Ortolani test (relocate dislocation) is positive at birth with the hip in a dislocated position at the beginning of the clinical examination, then this is again confirmed by US. Even some of these hips reduce spontaneously within 2 weeks without treatment. Therefore treatment can be held off for the first 2 weeks. Harness treatment is started if the hips do not reduce during this period.
In the harness the hips are flexed to 90° to 100° and allowed to abduct to 65°.
Treatment is continued until the US shows normal development of the hips. Most harness treatments go over 2 to 4 months. The harness is left on full time until the hip stabilises. The harness can be taken off for daily bath times thereafter. It is not used for children above the age of 6 months.
Pavlik harness treatment is abandoned if a hip does not reduce within 4 weeks of treatment. This also applies to bilateral dislocations where one hip remains dislocated at 4 weeks. Damage to the acetabulum occurs by directing the femoral head into the wrong position if treatment is continued. In these cases, an arthrogram is performed followed by closed reduction and spica immobilisation in most cases. An adductor longus release is sometimes added. Some surgeons replace the spica with a harness after one month when the hip has stabilised and continue with the harness until the hip looks normal. An open reduction is occasionally necessary.
Treatments and timings vary between orthopaedic surgeons. The hip development in the harness is monitored with regular US according to the surgeons preference.
Harness treatment is associated with an avascular necrosis rate of about 2% (up to about 8% has been reported). Femoral nerve palsy is rare.
2. 6 to 18 months of age
Patients are brought to theatre were an arthrogram of the hip joint is performed followed by either closed or open reduction with adductor longus release if it is contracted and hip spica application. Some surgeons perform an additional iliopsoas release. A CT or MRI scan is performed after reduction to confirm the position. The spica period is 3-4 months with a change half way through followed by immobilisation with a removable abduction brace for a further about 3 months.
The timing of the reduction is controversial. Some surgeons wait with the reduction for up to about one year.
A femoral derotation osteotomy is performed at times for those cases with excessive femoral neck anteversion.
3. Older than 18 months
Patients who present after the age of 18 months usually require an open reduction and hip realignment surgery. This includes femoral derotation osteotomy, pelvic osteotomy or both combined. An adductor longus release is generally necessary. Post-operative immobilisation is with a hip spica for 3 months. Bracing is usually not necessary after the spica period after realignment surgery.
The femur is derotated to reduce excessive femoral neck anteversion. It needs to be shortened if there is too much pressure on the acetabulum after reduction and varus needs to be incorporated if the femoral neck is in too much valgus.
The pelvic osteotomy most commonly performed is the Salter osteotomy but a Dega osteotomy is also an option.
Other pelvic reconstruction osteotomies used in older patients with DDH and other hip pathologies are: triple pelvic osteotomy (Steel, Tönnis), Ganz periacetabular osteotomy (Bernese). Pelvic salvage osteotomies are Chiari and Shelf procedure.
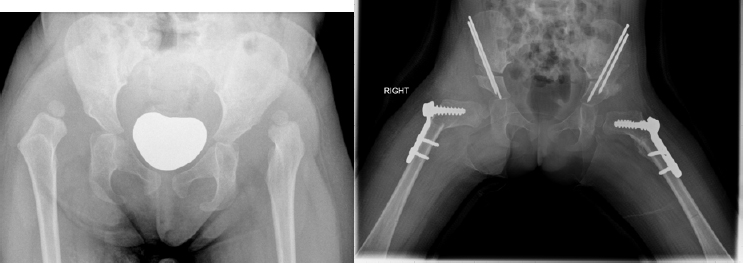
30 months old girl at first presentation.
Risk factors for DDH
NIPE = NHS Newborn and Infant Physical Examination programme
NIPE recommends:
A hip ultrasound should be performed when one or both of the following risk factors are present:
1) first degree family history of hip problems in early life
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree