Chapter 10 Overview of Biologically Based Therapies in Rehabilitation
Biologically based therapies are among the most popular forms of complementary and alternative medicine (CAM). The use of biologically based therapies is widespread in the United States and is increasing.1
CONSUMER USE OF BIOLOGICALLY BASED THERAPIES
Results from a recent survey by Kelly and colleagues1 that tracked weekly herb and dietary supplement use in 8476 American adults from 1998 to 2002 demonstrated that overall use of these biologically based therapies increased from 14.2% to 18.8%. Further, the results showed that herb and dietary supplement use in the older population doubled.1 In North America, a 2002 National Health Interview Survey (NHIS)2 interviewed more than 31,000 adult Americans and found high levels of CAM use when persons are healthy and sick.
Overall, the NHIS survey found that 36% of adults are using some form of CAM. When megavitamin therapy and prayer specifically for health reasons are included in the definition of CAM, that number rises to 62%.2 About one fifth of the adults surveyed by the NHIS used some form of natural product within the preceding 12 months. From the survey, CAM in general most often was used to treat and/or prevent musculoskeletal conditions or other conditions that involve chronic or recurring pain, issues particularly salient to care providers with clients needing rehabilitative services.2 Those more likely to use CAM therapies, including biological therapies, were women, those who had completed a higher level of education, adults who had been hospitalized recently, and former smokers.2
The use of CAM by parents or caregivers is the single best predictor of CAM use in children, and herbal use by children is on the rise, with 28% to 40% of children exposed to herbals for conditions such as asthma, anxiety, insomnia, and attention-deficit hyperactivity disorder (ADHD).3 In children with complex or chronic illnesses, such as cancer or autism, the rate of biological therapy use parallels that of adults with similar conditions, approaching 100%.
Trends in CAM use vary among different socioeconomic sectors and minorities in the United States. The NHIS data (Figure 10-1) provide some broad information on CAM use in general.
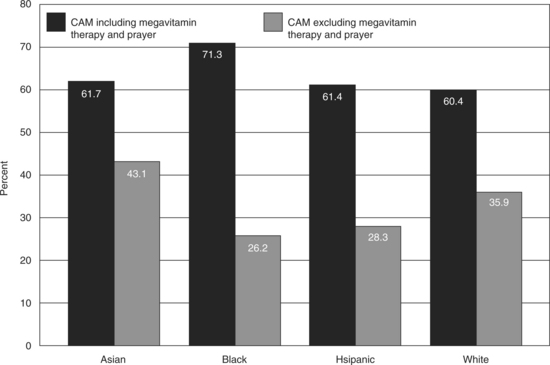
Figure 10-1 CAM use by race/ethnicity.
Source: from: http://nccam.nih.gov/news/camsurveyfs1.htm#natural
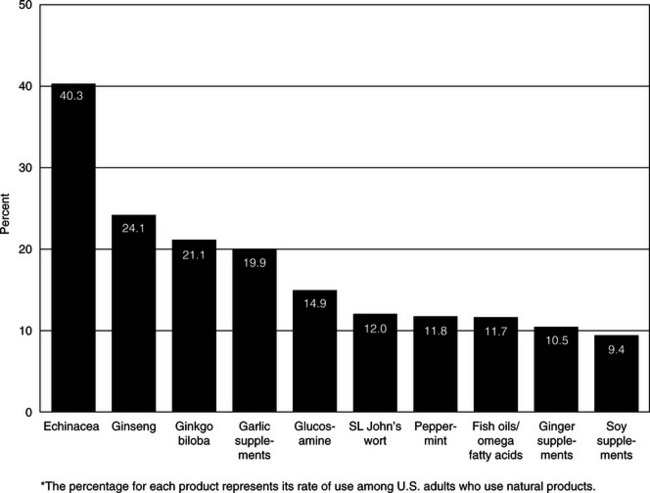
Research continues in the effort to characterize national trends in CAM use by minorities. To date, generalizations about CAM use are limited by geographical differences in demographics and influences between and among minority and ethnic communities. However, differences have been identified in limited or smaller scale studies. For example, in a survey of outpatient clinics in Houston, Texas, approximately half of Hispanics and half of Asians used herbs compared with 41% of Caucasians and 22% of African Americans.4 Also, clients were more likely to use herbs if they had an immigrant family history or if other family members used herbs. More Caucasians (67%) disclosed their herbal use to their health care providers than African Americans (45%), Hispanics, (31%), or Asians (31%).4 As of 2002, the top 10 botational or natural products used by American adults in the NHIS survey are identified in Figure 10-2.
Internationally, the use of botanical medicines is generally higher than in the United States. For example, 70% of “Western-trained” doctors in Japan prescribe kampo drugs daily.6 In Britain, recent investigations have shown that herbal medicine has been used by about 31% of the adult population.7 About 80% of the world’s population relies primarily on traditional medicines, including biologically based therapies, for their health care needs.8
KEY TERMS AND DEFINITIONS
According to the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (NIH), biologically based therapies “include, but are not limited to, botanicals, animal-derived extracts, vitamins, minerals, fatty acids, amino acids, proteins, prebiotics and probiotics, whole diets, and functional foods.”9 Clarification of these terms is key to understanding their uses, composition, safety, oversight, and regulation.
Botanical Products
Even for a particular class of product, a variety of terms may be used. Botanical drug products also may be known as herbal products or nutriceuticals. Botanical drug products are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in humans. A botanical drug product consists of vegetable materials, which may include plant materials, algae, macroscopic fungi, or combinations of these. These products may be available in a variety of forms, including a solution (e.g., tea), powder, tablet, capsule, elixir, topical, or injection. By distinction, fermentation products (e.g., alcohol) and highly purified or chemically modified botanical substances are not considered botanical drug products.10
Dietary Supplements
The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines a dietary supplement as a product (other than tobacco) taken in addition to a normal diet. More on this key legislation is mentioned below. Further, dietary supplements must contain one or more dietary ingredients (including vitamins; minerals; herbs or other botanicals; amino acids; and other substances such as enzymes, organ tissues, glandular materials, and metabolites). The U.S. Food and Drug Administration (FDA) oversees supplement use and regulation. The FDA characterizes and regulates botanicals and dietary supplements according to their use, not according to their composition. If the intended use is to “promote health,” the agent is viewed as a dietary supplement. By contrast if the intended application of a product is to treat or prevent a disease, the agent is considered to be a drug. To meet labeling requirements, a dietary supplement must be intended to be taken by mouth as a pill, capsule, tablet, or liquid.11
Herbs
An herb is the above-ground part of a nonwoody plant that does not persist through the winter. It also is known commonly as a crude drug, or as a flavoring used in cooking.12 Other terms are used synonymously for herbs or herbal products, including botanicals, nutriceuticals, and phytopharmaceuticals.
Prebiotics
Prebiotics are biologically based therapies used to alter the bacterial composition of the intestines, not by adding bacteria, but by changing the type of substrate provided to the existing mixture.13
Special Diets
Special diets, such as those advocated by Atkins, Ornish, Weil, and others, purport to prevent or mitigate the symptoms of illness and/or to promote wellness. Orthomolecular therapies include the use of specific types of ingredients, such as minerals, or high-dose vitamins to prevent or treat illness, or to enhance wellness.14
REGULATION AND SAFETY OF HERBS AND DIETARY SUPPLEMENTS
The DSHEA of 1994 has proven to be a landmark piece of legislation for the ongoing manufacture, safety, and use of biologically based therapies. Per this legislation, herbs and dietary supplements are regulated as foods rather than drugs. This means, unlike pharmaceuticals, herbs and dietary supplements are not required to meet the strict standards of safety, effectiveness, and quality established by the FDA. Knowing the distinction between the regulation of biologically based therapies and the regulation of pharmaceutical products has consequences for the consumer and clinician.
Effectiveness
Manufacturers do not have to prove that an herb or dietary supplement is effective. Rather, the manufacturer can say that the product addresses a nutrient deficiency, supports health, or reduces the risk of developing a health problem, if that is true. If the manufacturer does make a claim, it must be followed by the statement, “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”15
Quality
A manufacturer does not have to prove an herb or dietary supplement’s purity or caliber. The FDA is not required to analyze the content of herbal or dietary supplement products. Manufacturers who want to demonstrate a commitment to the quality of their products can voluntarily follow the FDA’s stricter—but optional—Good Manufacturing Practices or GMPs for drugs.15 The designation “GMP” on a supplement’s label ensures product quality, although it does not indicate safety or efficacy.
Adverse Events and Reactions
The MedWatch system of the FDA allows health care professionals and consumers to report serious adverse events, potential and actual product use errors, and product quality problems associated with FDA-regulated drugs, biologicals (including human cells, tissues, and cellular and tissue-based products), medical devices (including in vitro diagnostics), special nutritional products, and cosmetics. Reporting instructions and forms can be accessed at http://www.fda.gov/medwatch/how.htm
The MedWatch system has been used in limited fashion for adverse event reporting for herb/dietary supplement use since 2004.16 Websites for the Office of Dietary Supplements at the NIH17 contain searchable information on warnings and safety postings from the FDA and false advertising claims from the FTC. Other surveillance systems include information on biologically based therapies such as the American Association of Poison Control Centers (AAPCC) incident database known as the Toxic Exposure Surveillance System (TESS).18
SOURCES OF INFORMATION
Research
A number of issues have influenced the dissemination of information regarding the effectiveness and safety of biologically based therapies. The efficacies of herbal medicines that have been tested in clinical trials are of varying methodological quality. Fortunately or unfortunately, the gold standard of allopathic medicine for measuring the effectiveness of a therapy is the randomized controlled trial (RCT), yet RCTs on biologically based therapies are relatively limited in number. Despite an increased interest in biologically based therapies, research funding in this area is limited when compared with the budgets of the pharmaceutical sector. Even if many more RCTs on biologically based therapies were conducted, generalizing the data may be difficult because investigations may use several different botanical features and preparations to determine efficacy.19
As Ernst and others noted, analysis of the evidence for recent overviews of rigorous trials of herbal medicines has had mixed results: of 23 systematic reviews of herbal therapies, 11 came to a positive conclusion, nine yielded promising but not convincing results, and three were negative.20 Despite these inconsistencies, sensible recommendations can be made based on appraisal of the existing literature and rational risk-benefit analysis.
Commission E Monographs
In 1978 the German Ministry of Health established Commission E, a panel of experts charged with evaluation of the safety and efficacy of the herbs available in pharmacies for general use. The Commission reviewed more than 300 herbal drugs. This collection is known as the Commission E Monographs. The monographs are intended to provide guidelines for the general public, health practitioners, and companies applying for registration of herbal drugs. In general, they do not contain standards for assaying the quality and purity of herbal drugs, and they differ from North American and British publications in that some of their outcome measures do not use RCTs. For some clinicians and investigators, this may limit their use as a resource. Following the Commission E Monograph in Germany, the European Scientific Cooperative on Phytotherapy (ESCOP) evolved in Europe over the 1990s and reviews the therapeutic uses of herbal medicinal products. In all, 60 ESCOP monographs were published.21
National Center for Complementary and Alternative Medicine
In North America, the largest source of funding remains the NCCAM at the National Institutes of Health. NCCAM has declared that its future research portfolio will have an increased emphasis on studies of the mechanisms underlying CAM approaches, including biologically based therapies.22 For the busy clinician, the NCCAM web-site gives an overview of the government funded research in CAM (Box 10-1) and general information regarding and summaries of different forms of complementary therapies. For the health care provider or consumer, this Internet resource can be a helpful starting point to access general yet accurate and timely information about vitamins, minerals, herbs, and dietary supplements.
Natural Standard
Natural Standard, which can be accessed, online at http://www.naturalstandard.com/, is an international, multidisciplinary collaboration of clinicians and researchers founded to provide high-quality, evidenced-based information about complementary and alternative therapies. Natural Standard has developed several databases including Herbs & Supplements, Interactions, Brand Names, Condition Center, Complementary Practices, and Dictionary. For each therapy or botanical product included in the databases, an impartial research team has systematically gathered scientific data and expert opinions. Then, using a validated rating scale, the quality of the evidence is evaluated. Monographs written by the research team are then peer-reviewed before inclusion in the databases. The databases are organized so that a subscriber can read a comprehensive evidenced-based systematic review that provides details of efficacy, adverse effects, interactions, dosing, and more, or a simple “flashcard.” The flashcard has been adapted from a comprehensive review and provides a quick look- up and easy reading that can be used as a client handout. Another feature of Natural Standard’s databases is the “Condition Center-Comparative Efficacy Chart.” For these charts, medical conditions are cross-referenced across the evidence-based monographs and the interventions are arranged hierarchically by level of evidence for efficacy. Although Natural Standard appears to a valuable resource for user-friendly and highly credible information on complementary and alternative therapies, individual subscriptions to the databases are currently not available. According to the website, all database access and content licensing agreements need to be made between an organization, institution, or corporation.
USE OF BIOLOGICALLY BASED THERAPIES IN REHABILITATION MEDICINE
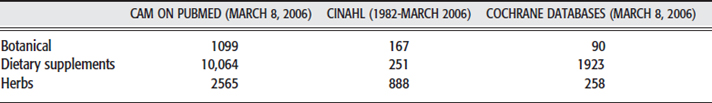
Approaching the literature search for botanicals used in rehabilitation medicine may yield very different results depending on the choice of search terms and the database selected. Table 10-1 demonstrates a representative literature search for the body of information presented below. CAM on PubMed provides the most fruitful source of information specific to the botanical approach to treating these conditions.
In this section, 20 common conditions for which clients seek rehabilitative therapy are addressed. The common herbs and supplements used for those conditions are listed. These botanicals have been classified based on the availability and quality of scientific evidence for their use in these conditions. The evidence for most of the botanicals listed has been evaluated in HealthNotes and classified into one of the three following categories:
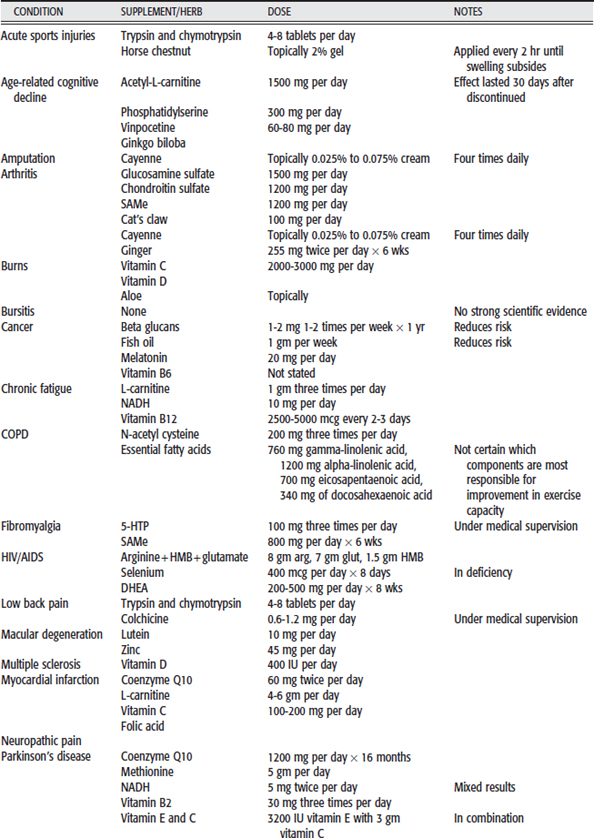
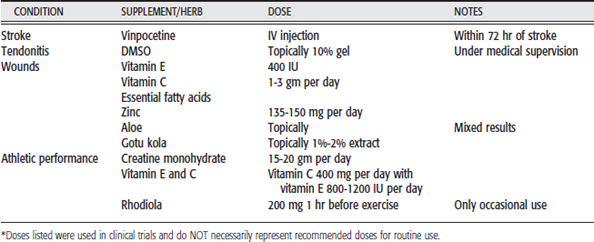
Herbs and supplements in the first category (reliable, consistent evidence) and the second category (contradictory or preliminary) are emphasized and placed in Table 10-2. Botanicals in the last category are deemphasized. Table 10-2 summarizes the herbs and supplements mentioned for the selected conditions. Doses and preparations used in clinical trials are listed. Special precautions or concerns are listed under Notes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree