Osteotomy of the Radius for Treatment of Kienböck Disease
Cameron T. Atkinson
Jeffrey E. Budoff
David S. Zelouf
DEFINITION
Kienböck disease is a disorder of undetermined etiology that results in avascular necrosis (AVN) of the lunate.7
ANATOMY
Lunate Vascularity
The extraosseous blood supply of the lunate is extensive: Branches of the radial and anterior interosseous arteries form a dorsal lunate plexus and branches of the radial, ulnar, and anterior interosseous arteries as well as the recurrent deep palmar arch form a volar plexus.
The intraosseous blood supply is variable. Because the lunate is covered by cartilage proximally and distally, vessels can enter the bone only at its dorsal and volar poles.2,16
Three studies have identified “lunates at risk” from a vascular standpoint. The vulnerable lunate is one that has large areas of bone dependent on a single intraosseous vessel, which occurs in 7% to 20%. In addition, 31% of lunates have no internal arterial branching.8,9,19 These internal vascular arrangements may render the lunate more vulnerable to AVN, as injury to the single vessel could not be compensated for by collateral flow.
Ulnar Variance
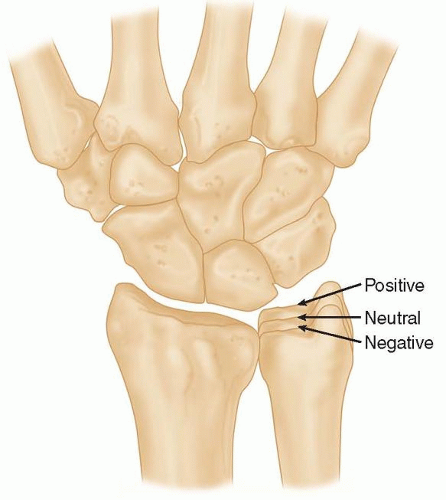
The standard posteroanterior (PA) wrist radiograph is taken with the shoulder and elbow at 90 degrees and the forearm in neutral rotation.
In this view, the length of the distal ulna with respect to the distal radius is called ulnar variance (FIG 1).
When the ulna is the same length as the radius, it is said to have neutral ulnar variance. When the ulna is shorter than the radius, it is referred to as negative ulnar variance, and when the ulna is longer than the radius, it is referred to as positive ulnar variance.
Theoretically, a negative ulnar variance increases shear forces on the lunate.
The triangular fibrocartilage complex (TFCC) is thicker in these patients and the difference in compliance between it and the ulnar edge of the radius is accentuated, leading to greater shear force.
In addition, loads across the radiocarpal joint are borne disproportionately by the radius.7
In the North American population, Kienböck disease is associated with a negative ulnar variance.
This relationship does not hold true in the Japanese literature.2
Other authors have noted a tendency toward smaller lunates in patients with Kienböck disease.3
PATHOGENESIS
The cause of Kienböck disease is incompletely understood. Current thinking is that acute or repetitive trauma causes excessive shear forces on a lunate at risk, interrupting its intraosseous vascularity and leading to AVN.1,2
Although a history of injury is elicited in over 50% of cases, the absence of a single traumatic event is still very common.
Fracture of the lunate has been reported in up to 82% of lunates with Kienböck disease.2 However, it remains unclear whether these fractures are the cause or the result of AVN.
Although transient ischemia may be seen after carpal fracture-dislocations, this spontaneously resolves after 5 to 32 months and should be treated expectantly.1,2
The key feature of transient ischemia is that no progressive radiographic collapse occurs, as opposed to Kienböck disease, where radiographic changes and collapse are predictable.
It has been suggested that Kienböck disease may be due to venous outflow obstruction with intraosseous vascular congestion rather than arterial insufficiency. Increased intraosseous pressure has been shown in lunates with Kienböck disease as well as in femoral heads with AVN.
This is more consistent with venous stasis than arterial compromise.
Once the lunate becomes avascular, stress fractures occur first in the proximal lunate adjacent to the radial articular surface, where the blood supply is poorest.2,8,16 Consequently, the proximal lunate is usually more involved and more flattened than the distal lunate. In addition, the radial lunate that articulates with the distal radius is usually more involved than the ulnar lunate that overlies the triangular fibrocartilage, probably because of the difference in compliance between the two supporting surfaces. This difference is accentuated in patients with negative ulnar variance.16
Lunate collapse leads to loss of carpal height. If a coronal plane fracture is present, the compressive forces of the capitate displace these two fragments volarly and dorsally.16
NATURAL HISTORY
The natural history of Kienböck disease is one of progressive fragmentation and collapse of the lunate, loss of carpal height with scaphoid flexion, and proximal capitate migration leading to perilunate arthritis. However, these changes do not universally lead to a poor clinical outcome.7
A follow-up study of 49 patients compared 23 wrists treated with mean 8 weeks of immobilization and 26 without treatment.14
In both groups, the majority reported a gradual decrease in symptoms over time.
At mean 20.5 years of follow-up, 83% of the wrists in the immobilized group were pain-free or were painful only with heavy work.
In the nontreated group, this was true for 77%.
In all wrists, the lunate was deformed and 67% developed radiocarpal arthritis on radiographs.
The authors concluded that Kienböck disease has a naturally benign course.
There was no correlation between residual symptoms and the radiographic appearance, including the appearance of arthritis.
In this study, immobilization did not lead to any long-term benefit.
PATIENT HISTORY AND PHYSICAL FINDINGS
Most patients with Kienböck disease are young, active patients between 20 to 40 years of age.
This has led to significant concerns about the long-term effects of this disorder.
Regardless of gender, more than 95% of patients are engaged in heavy manual labor.27
The most common complaints are dorsal central wrist pain, stiffness, and significant weakness of grip, which is often reduced to 50% of the opposite hand.2,7,16
There may be a long history of symptoms before presentation.
The pain may vary in intensity from mild discomfort to constant, debilitating pain. It is often activity related and improves with rest and immobilization.
The wrist is typically mildly swollen dorsally, consistent with synovitis, and is tender over the lunate.
Flexion and extension are predictably diminished.
Wrist flexion is more likely to be limited than extension because the volar pole of the lunate often extrudes so that it impinges against the volar rim of the distal radius.
Forearm rotation is not affected.16
Although Kienböck disease has been reported in association with steroid use, septic emboli, sickle cell disease, gout, carpal coalition, and cerebral palsy, there is no welldefined correlation with any systemic or neuromuscular process that warrants screening when considering the diagnosis.3
IMAGING AND OTHER DIAGNOSTIC STUDIES
Radiographic Classification
Kienböck disease is diagnosed radiographically, and staging is based on plain radiographs.
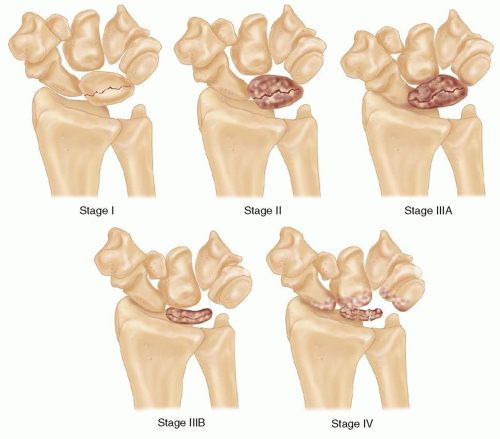
In 1977, Lichtman and Degnan15 modified Stahle’s original radiographic classification in an attempt to help guide treatment decisions (FIG 2).
Stage I
Radiographs are normal, although a linear fracture without sclerosis or lunate collapse is occasionally present.
Stage II
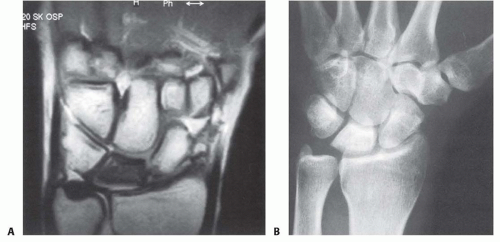
The lunate becomes sclerotic and radiodense, similar to the radiologic appearance of other bones with AVN (FIG 3B). A coronal fracture splitting the lunate into dorsal and volar fragments may be noted.
Late in stage II, some loss of lunate height on the radial side may be evident.
Stage III
The lunate collapses in the coronal plane and elongates in the sagittal plane. The carpal architecture is altered and the capitate begins to migrate proximally.
Stage IIIA
Lunate collapse has occurred, but carpal height is relatively unchanged and carpal collapse has not yet led to proximal migration of the capitate or scaphoid flexion. Therefore, the carpal kinematics have not yet been significantly altered.
Stage IIIB
Stage IV
Magnetic Resonance Imaging and Computed Tomography
MRI is extremely sensitive in detecting changes in marrow fat that are consistent with, but not diagnostic of, AVN.
Decreased signal on T1 sequences represents replacement of the normal fatty marrow by dead bone or fibrous tissue.27
Because MRI detects only the loss of marrow fat and not AVN specifically, to consider an MRI diagnostic for Kienböck disease, over 50% of the lunate should be hypointense on T1 because the changes of Kienböck disease are diffuse, as opposed to other conditions such as ulnocarpal impaction, fractures, and intraosseous tumors, which cause more focal MRI changes.4,25,28
It is possible that a large enchondroma, interosseous ganglion, or other marrow-replacing lesion could lead to MRI changes in over 50% of the lunate. Thus, there is currently no truly pathognomonic imaging sign for Kienböck disease.4
T2 images typically show low signal intensity, which represents replacement of the normal fatty marrow by fibrosis.27
An increased T2 signal may occur if intramedullary edema is present or if revascularization is occurring.3,4,25 Thus, when the T2 images show normal or increased signal intensity, an earlier stage of disease with a better prognosis can be inferred.25,27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree