Osteoporosis
Osteoporosis is a systemic skeletal disorder characterized by low bone mass, disruption of the microarchitecture of bone tissue, and compromised bone strength that leads to an increased risk for fracture.
Osteoporosis is common among menopausal women but is usually clinically silent until a fragility fracture occurs. Hip fractures are the most devastating of these in terms of medical, psychosocial, and financial consequences. The lifetime probability of sustaining a hip fracture for a 50-year-old white woman is 14%. The overall percentage of white women at age 50 or older who will sustain an osteoporotic fracture in their remaining lifetime is ∼40%. Osteoporosis is being recognized with increasing frequency in older men, who account for about one third of all hip fractures in the United States. The 1-year mortality of men after a hip fracture approaches 30%. Finally, patients receiving long-term glucocorticoid therapy are at increased risk for osteoporosis and should have prevention and treatment approaches implemented.
- Reduced bone mineral density.
- Decreased bone strength.
- Fragility fractures.
Bone loss in women begins before the onset of menopause, typically in the late third and early fourth decades, and then accelerates for the 5–10 years after the menopause. Postmenopausal osteoporosis is thought to result from an estrogen-deficiency–induced imbalance between bone formation and resorption such that resorption predominates over formation. Following the increased rate of bone loss immediately surrounding the menopause, a less aggressive phase of bone loss ensues that continues into the eighth and ninth decades. Estrogen deficiency and factors related to aging (reduced osteoprogenitor population, nutritional deficiencies, and malabsorption) play a role in this later phase of bone loss.
Clinically, osteoporosis is diagnosed when bone mineral density (BMD) is reduced or when fragility fractures occur. Such fractures are operationally defined as occurring after little or no trauma such as falling from a standing height. The most common osteoporosis-related fractures involve the thoracic and lumbar spine, hip, and distal radius.
Bone densitometry by dual energy x-ray absorptiometry (DXA) is the best standardized technique for diagnosing osteoporosis and monitoring responses to therapy. DXA assessments have been used by the World Health Organization (WHO) to define osteopenia and osteoporosis. Their criteria are based on a large body of data on postmenopausal white women (Table 58–1).
| Definitions | |
|---|---|
| T-score | Number of SD above or below peak bone mass (“young normal”) according to race or ethnicity |
| Z-score | Number of SD above or below age-matched bone mass according to gender and race or ethnicity |
| Normal | BMD T-score ≥ –1 |
| Low bone mass (osteopenia) | BMD T-score < –1 and > –2.5 |
| Osteoporosis | BMD T-score ≤ –2.5 |
| Severe osteoporosis | BMD T-score ≤ –2.5 with one or more fragility fractures |
In addition to age and BMD, there are other risk factors associated with an increased incidence of osteoporotic fractures; the National Osteoporosis Foundation (NOF) has categorized these as modifiable and nonmodifiable (Table 58–2). As described below, all treatment and prevention strategies for osteoporosis begin with risk factor assessment and modification.
|
Until recently, there was no validated instrument for combining clinical risk factor assessments with BMD measurements to obtain absolute fracture risk estimates. In 2008, the WHO released its fracture risk calculator called FRAX (www.sheffield.ac.uk/FRAX). The FRAX tool combines gender and ethnicity together with several clinical risk factors (age, prior fracture history, parental hip fracture, current smoking, long-term use of glucocorticoids, rheumatoid arthritis, and excessive alcohol consumption) in combination with femoral neck or total hip BMD to generate two probabilities: (1) 10-year absolute risk of hip fracture, and (2) 10-year absolute risk of major osteoporotic fracture (hip, spine, wrist, and humerus). The clinical risk factors used in FRAX calculation can be easily ascertained during the initial evaluation of the patient. NOF adapted FRAX for use in US women and men of different ethnicities (white, black, Hispanic, Asian) and set new treatment thresholds based on absolute fracture risk and cost-effectiveness analysis. Since its introduction, FRAX has been modified and updated. Their recommendations are summarized in Table 58–3. Recommendations for the use of BMD testing in postmenopausal women and for patients receiving glucocorticoids are in Table 58–4.
|
|
|
|
|
The evaluation of perimenopausal or postmenopausal women with osteoporosis or a low BMD begins with the clinical assessment. At least 10–20% of postmenopausal women have an additional secondary cause for their bone loss beyond the estrogen deficiency of menopause. When taking the medical history, the practitioner should pay careful attention to medication use (especially glucocorticoids), smoking, alcohol intake, dietary calcium intake, and family history of osteoporosis and fractures. The physical examination focuses on height loss, the presence of bone pain or deformity, and signs of anemia, hyperthyroidism, hypercortisolism, malnutrition, and other disorders that cause secondary forms of osteoporosis (Table 58–5).
| Rheumatologic disorders |
|
| Connective tissue disorders |
|
| Endocrine disorders |
|
| Hematologic disorders |
|
| Gastrointestinal disorders |
|
| Other conditions |
|
| Drug therapy |
|
There is no consensus about the appropriate and cost-effective laboratory work-up for postmenopausal women with low BMD or osteoporosis. At a minimum, however, laboratory evaluation should include a complete blood cell count, serum chemistry panel, liver function tests, and serum thyroid-stimulating hormone and calcium determinations. Consideration should be given to assessing vitamin D status at the outset, and this is done by measuring 25-hydroxyvitamin D levels.
Postmenopausal women as a group are commonly affected by primary hyperparathyroidism (prevalence ∼3 per 1000). Serum calcium and albumin determinations adequately screen for this disorder. In addition, multiple myeloma can be relatively silent clinically and present with osteoporosis, bone pain, pathologic fractures, or anemia. This diagnosis should be considered if BMD is remarkably low for age (ie, a low Z-score) or if low BMD is accompanied by an unexplained anemia or an elevated erythrocyte sedimentation rate. Multiple myeloma can be detected by serum and urine protein electrophoreses.
Measurements of serum 25-hydroxyvitamin D and urinary calcium excretion can be very helpful. Subtle vitamin D deficiency is relatively common in elderly patients and contributes to bone loss because it interferes with the absorption of calcium and phosphorus and thus the mineralization of bone matrix. Low urinary calcium excretion can be caused by vitamin D deficiency, but it can also be due to underlying malabsorption or an extremely low calcium intake. Subtle forms of calcium malabsorption (eg, secondary to celiac sprue) are more common than previously thought. High levels of urinary calcium excretion suggest idiopathic hypercalciuria, which can be associated with renal stones or low BMD or both. Urinary calcium measurements in a patient taking calcium supplements can be informative as to absorption and the adequacy of therapy.
The study of choice to assess BMD in a postmenopausal woman is DXA of the lumbar spine and hip. DXA reports often include both T- and Z-scores (Figure 58–1). A T-score relates the BMD of the patient to peak bone mass for race and gender. A Z-score relates the BMD of the patient to persons of the same age, gender, and race. The T-score is the more useful determination clinically. Operationally, the lower of the two T-scores (spine or hip) is used for making the diagnosis. Typically, there is concordance between T-scores at both sites. Discordance can be due to artifactual elevation of the spinal measurement as a result of degenerative arthritis, disk disease, or aortic calcification; in such cases, only measurements of the hip and femoral neck should be used.
Figure 58–1.
This graph shows the mean ± 1 standard deviation for bone mineral density (BMD). For a given femoral neck BMD value in a 70-year-old woman, her Z-score is –1 and her T-score (extrapolated back to the age of 20) is –2.4. (Used, with permission, from Orwoll ES, Bliziotes M. Osteoporosis: Pathophysiology and Clinical Management. Humana Press, 2003:109.)
Obtaining a BMD measurement allows the clinician not only to grade the severity of osteoporosis but also to assess fracture risk. Several studies have confirmed that the relative risk of fracture approximately doubles with each standard deviation below peak BMD (ie, a negative T-score) that a patient demonstrates.
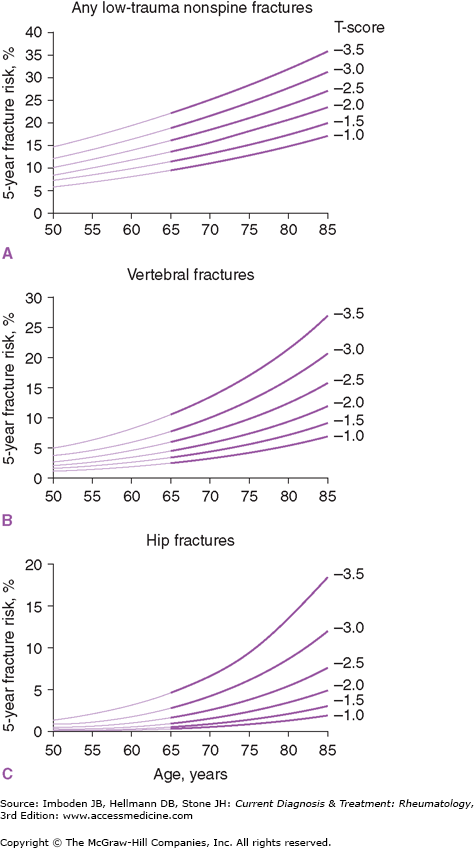
The combination of BMD measurement and the patient’s age is an even more powerful predictor of fracture risk. The 10-year risk of several types of osteoporotic fractures is strongly related to age and BMD T-score, and age is a powerful risk factor in the FRAX calculator of absolute fracture risk (Figure 58–2). Advanced age (over 70 years) dramatically increases the risks of vertebral and hip fractures.
Figure 58–2.
These figures show the influence of age and bone mineral density (BMD) on the 5-year fracture risk for any low-trauma non-spinal fracture (A), vertebral fractures (B), and hip fractures (C). At approximately age 65, there is a steep increase in fracture risk at T-scores of –2.0 or –2.5 or greater in panels B and C. (Used, with permission, from Cummings SR, Bates D, Black DM. Clinical uses of bone densitometry: scientific review. JAMA. 2002;288:1889.)
Postmenopausal osteoporosis can progress silently over years to a dangerously low BMD levels thereby reducing bone strength such that fracture threshold is reached. At this stage, fragility fractures can occur with minimal impact. Fractures are the dreaded complication of osteoporosis. Fractures of the spine cause pain that is generally self-limited. Multiple vertebral fractures can lead to loss of height, reduced thoracic expansion capacity and difficulty with breathing, progressive thoracic kyphosis, poor functional status, and ultimately increased frailty. Frailty itself is a risk factor for fractures, thereby setting up a vicious cycle.
Hip fractures have a more dramatic course, and prognosis in the elderly osteoporotic patient is guarded. These fractures require hospitalization and surgery. Because of the underlying frailty of most of these patients, their comorbid conditions and advanced age, and the prolonged immobilization and rehabilitation required, patients who fracture their hips have decreased life expectancy. Overall, mortality in the first year after a hip fracture (men and women combined) is approximately 20%.
In addition to the medical and financial consequences of hip fractures, there are substantial long-term human consequences. Hip fractures are life-altering events. It is estimated that 50% of patients who sustain a hip fracture do not live independently afterwards. Therefore, it is important to recognize osteoporosis early and to intervene with treatment strategies that reduce fracture risk.
- Usually presents later in life than postmenopausal osteoporosis.
- Fragility fractures, height loss.
- Most patients have one or more secondary cause of bone loss.
The diagnosis of osteoporosis in men is usually delayed relative to that of postmenopausal osteoporosis and often is made only after the patient presents with fractures, height loss, or obvious stigmata of the secondary causes for bone loss.
In contrast to women, men do not experience a clear-cut, easily defined cessation of gonadal function that raises awareness of risks for bone loss. Testosterone production declines with age, but there is controversy as to what the normal or accepted ranges of testosterone are for elderly men and how age-related declines in testosterone contribute to age-related declines in BMD. It is clear, however, that replacing testosterone does not restore BMD to “normal” even in hypogonadal men and that there are many determinants of low BMD and fractures in men beyond hypogonadism.
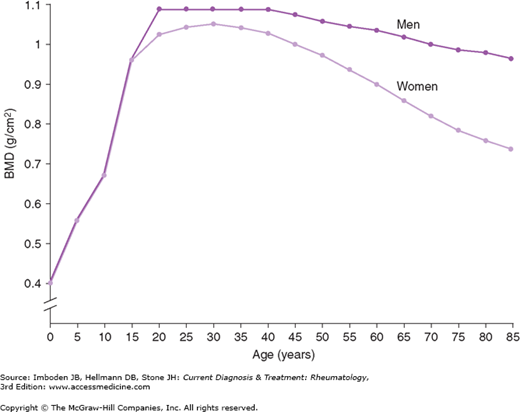
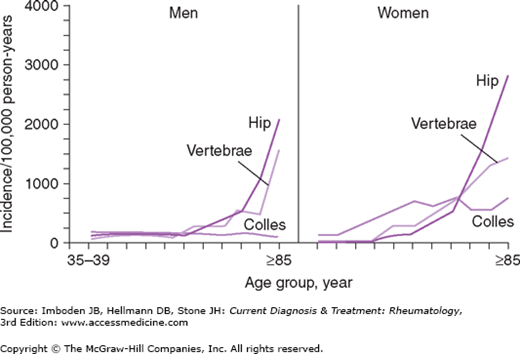
Differences in the peak bone mass and the rate of bone loss influence the differential clinical expression of osteoporosis in men and women. Men achieve higher peak bone mass than do women, and men lose bone mass at different rates than do postmenopausal women. In women, once the early rapid phase of postmenopausal bone loss ends, the rate of bone loss slows. Thereafter, the rates of bone loss in men and women are roughly comparable (Figure 58–3). Because of the early rapid phase of menopause-related bone loss and the lower peak bone mass attained by women, women have lower BMDs than men of the same age. This leads to an earlier onset of the typical osteoporotic fractures (hip, vertebral, and Colles fractures) in women compared with men (Figure 58–4). Men with low BMD experience most of the same fractures, but these fractures occur later—approximately 10 years later on average—than they do in women. Osteoporotic fractures therefore tend to occur in men at stages in their lives when they are more likely to be frail and are less able to cope physically and emotionally with the loss of independence and the risks of fracture repair surgery. Hence, the morbidity and mortality of hip fractures are much greater in men than in women.
Figure 58–3.
Mean bone mineral density (BMD) in men versus women from age 5 to 85 demonstrating the lower peak BMD values for women versus men, the rapid perimenopausal rates of bone loss in women, and the slow continuous phase of bone loss that continues into the eighth decade. (Used, with permission, from Southard RN, Morris JD, Mahan JD, et al. Bone mass in healthy children: measurement with quantitative DXA. Radiology. 1991;179:735; and from Kelly TL. Bone mineral reference databases for American men and women. J Bone Miner Res. 1990;5(Suppl 2):702.)
At least 80% of men with osteoporosis have one or more secondary causes of bone loss (Tables 58–5 and 58–6). Only 10–20% of men with low BMD have primary osteoporosis. These men are often middle-aged and present with height loss and multiple fractures. Whether they have intrinsic abnormalities in bone remodeling—defects in formation or resorption—is unknown.
|
Considerable effort has been directed toward identifying additional clinical risk factors for BMD loss and fractures in men as they age. An analysis from the MrOS study, a prospective cohort of 5995 elderly men (≥65 years old) from six US cities followed over 4.1 years at the time of the report is summarized in Table 58–7. Investigators found that six clinical risk factors were significantly associated with risk of hip fracture independent of hip BMD by DXA. Having three or more of these risk factors increased a man’s risk of fracture approximately 5-fold. Adding a low BMD to the risk factor profile—three or more risk factors plus being in the lowest BMD tertile—led to a 15-fold greater risk than the reference group (men without risk factors in the highest BMD tertile). There have been studies in countries outside the United States (Sweden, China, and so forth) that extend this type of prospective cohort analysis in men, but much still remains to be learned about susceptibility to fracture in men.
| Risk Factor | Hazard ratio (HR) from multivariable analysis with 95% confidence interval (CI) |
|---|---|
| Depression | 1.72 (95% CI, 1.00–2.95) |
| Tricyclic antidepressant use | 2.36 (95% CI, 1.25–4.46) |
| Previous fracture (>age 50) | 2.07 (95% CI, 1.62–2.65) |
| Inability to do a narrow walk trial | 1.70 (95% CI, 1.23–2.34) |
| Experienced a fall in the prior year | 1.59 (95% CI, 1.23–2.05) |
| Age ≥80 years | 1.33 (95% CI, 1.01–1.76) |
Because of the prominence of secondary forms of osteoporosis, the clinical and laboratory investigation of men with low BMD and fractures must be thorough. The history and physical examination should pay attention to possible underlying pulmonary, gonadal, adrenal, gastrointestinal, and hematologic disorders. Habits (alcohol and tobacco use) are important risk factors to pursue in men together with the signs and symptoms of the disorders listed in Table 58–5. Men with a history of prostate cancer should be asked about past (or current) use of long-acting gonadotropin-releasing hormone agonists and androgen blockers. These men lose bone at an accelerated rate and treating them to prevent bone loss is effective. Such treatment suppresses turnover, halts loss, and reduces future fractures.
The complete, cost-effective laboratory evaluation of men with osteoporosis is unknown. Most would agree that the initial evaluation should include a complete blood cell count; liver function tests; determinations of serum chemistries, testosterone, thyroid-stimulating hormone, calcium, phosphate, and 25-hydroxyvitamin D; and 24-hour urinary calcium excretion.
If anemia is present and no other cause for osteopenia or osteoporosis has been identified, serum and urine protein electrophoreses should be performed to exclude multiple myeloma. This diagnosis should always be considered in older black men with unexplained bone loss because of its predilection for that population. Men with low testosterone levels should be referred to an endocrinologist for evaluation for primary or secondary forms of hypogonadism.
While primary hyperparathyroidism is a key consideration in women with low BMD, it is less prominent as a cause of accelerated bone loss in men. Nonetheless, because it is relatively common, it should be considered if there is a history of renal stones or if the serum calcium value is even marginally elevated.
Hypercortisolism is rare, but bone loss may be the very first clinical clue that cortisol excess is present. Cushing syndrome is easily excluded by a 24-hour urinary cortisol determination or overnight dexamethasone suppression testing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree