Chapter 36 Osteochondral Grafts
Diagnosis, Operative Techniques, and Clinical Outcomes
INTRODUCTION
Hyaline articular cartilage is an avascular and insensate tissue that allows low-friction transmission of physiologic loads in diarthrodial joints. Its functional structure ideally is maintained in homeostasis over the lifetime of an individual, but remains incapable of mounting an effective repair response when injured in the skeletally mature adult.10,35 The treatment threshold for surgical intervention is not unequivocal, but patients with symptomatic lesions are generally considered candidates for cartilage restoration procedures.3
The use of osteochondral grafts of autologous or allogeneic origin is well supported on a basic science level and has a long successful clinical history as a means of biologic resurfacing.9,16 Both modalities rely on transplanting mature hyaline cartilage containing viable chondrocytes attached to subchondral bone to restore the architecture and characteristics of native tissue in acquired osteoarticular defects. By transplanting structurally complete osteochondral units with an intact tidemark, the fixation issue is mostly relegated to that of osseous ingrowth.31 Whereas both graft sources represent a common cartilage organ transplantation paradigm and are complementary, each has its unique, reciprocal challenges with regard to tissue availability and safety that must be weighed, managed, and communicated whenever the use of osteochondral grafts is being considered.
INDICATIONS
Autografts
In the authors’ hands, autologous grafts are best used to address relatively small, yet symptomatic, focal articular lesions of the femoral condyles, especially if these present with associated subchondral abnormalities such as a bone cyst or an intralesional osteophyte. Autologous plugs are also a potential salvage option as in situ fixation for a delaminating osteochondritis dissecans (OCD) lesion (International Cartilage Repair Society [ICRS] grade II–IV, see Chapter 47, Articular Cartilage Rating Systems).19,41
Advantages of autologous grafts are immediate availability, relatively low cost, and their nonantigenic and osteogenic behavior that routinely lead to reliable osteointegration.11 The possibility of arthroscopic delivery of these smaller grafts is appealing, albeit technically challenging.
One obvious disadvantage of autologous graft sources is that the maximum graft surface area is self-limited by donor volume or lack thereof, for small and at most medium-sized lesions. This is especially true in the previously injured and/or operated knee, in which suitability regarding tissue quality and overall joint topography has to be critically assessed. In addition, donor-site morbidity can significantly add to the disease burden during intra-articular transfer.9
Allografts
Osteochondral allografts are ideally suited to treat medium to large chondral and osteochondral lesions.24 In particular, osteochondral allografting may be considered the current “gold standard” treatment for high-grade (ICRS grade III–IV) OCD about the knee. Other specific conditions amenable to allografting include osteonecrosis and post-traumatic defects, such as after periarticular fractures. Further indications for allografting of the knee include treatment of patellofemoral chondrosis or arthrosis and highly select cases of multifocal or bipolar post-traumatic or degenerative lesions. In a case in which meniscus replacement is also necessary, a composite tibial plateau with attached meniscus can be transplanted. Allografts are also increasingly employed in the salvage of knees that have failed other cartilage resurfacing procedures. Primary treatment may be considered in large chondral defects, whose size presents a relative contraindication for other treatments and for which the surgeon believes other procedures may be inadequate.
Osteochondral allografting is the only treatment option that restores mature orthotopic hyaline cartilage and reproduces the site-appropriate anatomy of the native joint both macroscopically and microscopically, without the risk of inducing donor-site morbidity.23 Osteochondral allografts are versatile addressing even very large, complex, or multiple lesions in topographically challenging environments, especially if they involve an osseous component.
Obvious drawbacks to the allograft paradigm are the relative scarcity of donor tissue, financial and logistical issues of graft procurement, and residual risk of infection, a discussion that is an essential part of the informed consent process.24 Although rare allograft-associated bacterial infections have been reported,49 there are no available published data quantifying this risk or that of viral transmission. Patients are counseled that the risk for disease transmission from a fresh osteochondral allograft is comparable with that associated with banked blood transfusion. In a 30-year experience at the authors’ institution, using over 500 fresh allografts, no cases of transmission of disease from donor to recipient have been documented.
Fresh, cold-stored osteochondral allografts have shown to maintain viable chondrocytes and mechanical properties of the matrix many years after transplantation.4,14–16,30,38,52 These findings have generally supported the use of tissue for small osteochondral allografts in the setting of reconstruction of chondral and osteochondral defects. Chondrocyte viability and structural integrity of the matrix are preserved during hypothermal storage in nutritive culture medium containing human serum, with cell density, viability, and metabolic activity remaining essentially unchanged from baseline for as many as 14 days before deteriorating significantly after 28 days while the hyaline matrix remains relatively intact.6,45,47,50,53 The clinical consequences of these storage-induced graft changes have yet to be determined, but 28 days is generally considered the threshold of graft utility in present clinical practice.
CLINICAL EVALUATION
A careful and focused history and physical examination are essential to determine candidacy for any cartilage restoration procedure. Because articular cartilage itself is insensate, it is important to identify contributory pain generators and to distinguish them from mechanical symptoms. Tools such as the ICRS Cartilage Injury Evaluation Package, which incorporates several validated outcome measures, can be helpful in systematically documenting the anatomic condition and functional envelope of the knee and in establishing a baseline for therapy.1
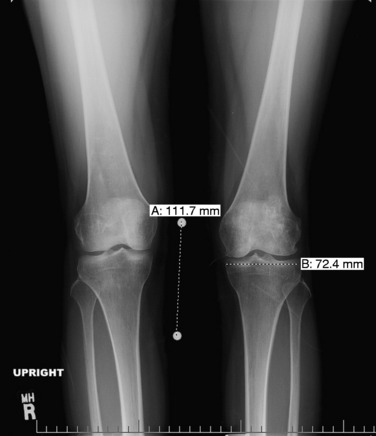
Patients who are considered for an osteochondral grafting procedure should optimally be fully imaged, including a complete radiologic series and magnetic resonance imaging (MRI) studies with cartilage-specific sequencing, if available. Depending on the type and location of cartilage injury being suspected, this should include at least standing anteroposterior (AP) weight-bearing and flexed knee lateral radiographs. Many chondral lesions and their subchondral sequelae are detectable on the AP views (Fig. 36-1), which also give an indication of possible secondary changes such as joint space narrowing and osteophytes. Imaging both knees side-by-side allows for a built-in comparison view. Of note, OCD presents with bilateral lesions in about a third of cases that are often easily detected on an x-ray in their typical location on the lateral aspect of the medial femoral condyle toward the intercondylar notch. The lateral flexed view is an important supplementary tool to help assess lesion size, triangulate locations, and identify patient morphology such as patella alta or baja and trochlear groove topography. Additional views that can be obtained include standing posteroanterior 45° flexed knee (Rosenberg) views and supine flexed knee (Merchant) views. The Rosenberg view brings the posterior condyles into view, which helps assess the posterior joint space during loading. Merchant views are standard for the evaluation of the patellofemoral articulation. Long-leg (hip to ankle) standing AP weight-bearing films should be obtained if axial alignment is deemed contributory to the patient’s symptomatology and are essential for preoperative planning if a realignment procedure is being considered.
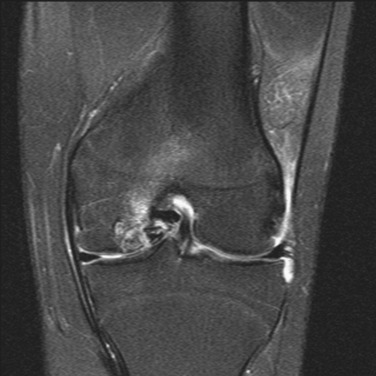
MRI remains an invaluable tool for assessing the status of the articular cartilage and associated structures of the knee46 (Fig. 36-2). While T2-weighted relaxation time maps are currently recognized as the gold standard for imaging the ultrastructure of the articular cartilage, a standard fat-saturated T2-weighted fast-spin-echo sequence usually conveys sufficient detail on cartilage morphology to guide surgical decision-making and preoperative planning. T1-weighted sequences are generally better suited to assess involvement of the subchondral bone and menisci.
PREOPERATIVE PLANNING
Common to all fresh allografting procedures is matching the donor with the recipient. It should be noted that in current practice, small-fragment fresh osteochondral allografts are not human leukocyte antigen– (HLA-) or blood type–matched between donor and recipient and that no immunosuppression is used. The allografts are matched to recipients on size alone. In the knee, an AP radiograph with a magnification marker is used (see Fig. 36-1), and a measurement of the mediolateral dimension of the tibia, just below the joint surface, is made and corrected for magnification of the radiograph. This corrected measurement is used, and the tissue bank makes a direct measurement on the donor tibial plateau. Alternatively, a measurement of the affected condyle can be performed.28 A match is considered acceptable at ± 2 mm; however, it should be noted that there is a significant variability in anatomy, which is not reflected in size measurements. In particular, in treating OCD, the pathologic condyle is typically larger, wider, and flatter; therefore, a larger donor generally should be used.
Critical Points GENERAL SURGICAL PRINCIPLES OF ALLOGRAFTING
INTRAOPERATIVE PLANNING
Autografting
Historically, the intercondylar notch and lateral trochlea were presumed to be non–load-bearing and thus recommended as donor sites for autologous osteochondral grafting. More recent reports have demonstrated that these areas do bear significant weight, which can theoretically contribute to increased donor-site morbidity. The lateral trochlea appears to be the most involved in loading, followed by the intercondylar notch and the distal medial trochlea.2 Because the lateral trochlea is wider than the medial side, the medial trochlea may best be suited for smaller donor plugs (<5 mm).20 Larger plugs can be taken from the lateral trochlea, starting proximal to the sulcus terminalis, where the lowest contact pressures of the lateral trochlea were measured. Owing to its load-bearing demands, the lateral trochlea appears to have the thicker articular cartilage, making it the favorite graft source of most surgeons. Plugs taken from the far medial and lateral margins of the femoral trochlea, just proximal to the sulcus terminalis, also appear to provide the most accurate reconstruction of the surface anatomy of central lesions in the weight-bearing portion of either femoral condyle.7
Smaller grafts (4 or 6 mm) from the lateral intercondylar notch can also provide precise matches to similar lesions; however, significant inaccuracies are noted when the lateral intercondylar notch grafts are increased in size (8 mm). Whereas all traditional donor sites have less cartilage thickness than common recipient sites, this discrepancy is most profound between the lateral intercondylar notch and the weight-bearing portion of the femoral condyles.48 In addition, the concave central intercondylar notch grafts do not match the topography of the convex femoral condyles,2 and their harvest jeopardizes the integrity of the trochlear subchondral bone, which might be responsible for the increased incidence of anterior knee pain in some studies.
OPERATIVE TECHNIQUE
Autografting
Biomechanical studies have confirmed that optimally sized, level-seated plugs face nearly normal joint contact pressures in situ.34 Results also show that slightly countersunk grafts and angled grafts with the highest edge placed flush to neighboring cartilage demonstrate fairly normal contact pressures. Conversely, elevated angled grafts increase contact pressures as much as 40%, making them biomechanically disadvantageous.33 The general consensus is that it is more favorable to leave a graft slightly countersunk than elevated with respect to the neighboring cartilage.42 The authors thus advise slightly lengthening the recipient tunnel to avoid leaving the graft proud or subjecting it to undue insertion forces in an effort to bury an oversized graft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree