Orofacial Infections
Thomas R. Flynn
Joseph F. Piecuch
Richard G. Topazian
Most infections of the oral cavity and face in children are odontogenic in origin and often are responsive to local treatment. Occasionally, spread to adjacent or distant fascial spaces or to the maxilla and mandible may result in life-threatening complications.
ODONTOGENIC INFECTIONS
Pathogenesis
Normal Flora of the Oral Cavity
The oral cavity provides an environment favorable to the growth of microorganisms. Bacterial counts in the range of 108 to 1,011/mL of saliva have been reported. Normally, more than 30 species of bacteria can be identified in saliva in varying proportions, depending on a dynamic interaction of different microbial ecosystems, including the tongue, the gingival crevice, and the presence of bacterial plaque. Variously, factors that can modify the microbial population include age, anatomic relationships, eruption of teeth, presence of decayed teeth, diet, oral hygiene, antibiotic therapy, systemic disease, and hospitalization. The estimated ratio of anaerobic to aerobic organisms in the oral cavity ranges from 3:1 to 10:1. Even the edentulous person may have a preponderance of anaerobes, because such areas as the buccal vestibule, when the cheek is approximated against the teeth or alveolar ridges, may have greatly reduced oxygen tension.
The flora of children is fairly similar to that of adults, with several exceptions. At birth, the oral cavity is sterile, but colonization with a wide variety of microbes occurs within hours. Although many sources of oral microbial colonization exist, a predominant source has been shown to be the oral flora of infants’ mothers. Interestingly, the bacterial strains found in fathers’ mouths are not found routinely in children’s oral cavity. Streptococcus salivarius has been found in 80% of cultures taken from 1-day-old infants. The percentage of Streptococcus species decreases from 98% on the day after birth to 70% at age 4 months as other organisms become established. Staphylococcus species, Neisseria, Veillonella, Actinomyces, Nocardia, Fusobacterium, Bacteroides, Corynebacterium, Candida, and a variety of coliforms gradually become established by age 1 year. As eruption of the primary dentition occurs, anaerobic organisms become well established in the gingival crevice, yet spirochetes and the Bacteroides melaninogenicus group (now known as the Prevotella and Porphyromonas genera and commonly associated with the gingival crevice in adults) appear to be present in fewer numbers before ages 13 to 16 years.
Pathogenic Organisms
Most odontogenic infections, whether they are carious, periodontal, or periapical, are polymicrobial, with a mixed aerobic-anaerobic flora averaging four to six isolates per case. A combination of an oral streptococcus and an anaerobe is involved in the majority of these infections. Early in the course of an infection, the streptococci predominate, invading tissue and spreading infection by elaborating enzymes, such as hyaluronidases, that break down the ground substance of connective tissue. This process generates necrotic tissue and a reduced oxygen environment and provides such nutrients as vitamin K, hemin, and succinate that favor the growth of oral anaerobes, including Prevotella, Porphyromonas, Fusobacterium, and anaerobic streptococci. As the infection matures, the anaerobes cause tissue liquefaction via collagenases, producing an abscess with a mixed flora. Late infections, with chronic encapsulated abscesses, often yield a purely anaerobic culture. The primary pathogens in orofacial odontogenic infections are identified in Table 247.1.
The taxonomy of the oral flora is changing. Clinical isolates from orofacial infections still may be identified by the laboratory as Streptococcus viridans or B. melaninogenicus; however, research involving genetic analysis of the various strains of these species has resulted in a rapidly changing classification and nomenclature for these organisms, with the acceptance of several new genera and species within these older classifications. Current classifications are summarized in Table 247.2. As molecular diagnostic methods develop, our understanding of the roughly 60% of oral species that cannot be cultured will change significantly.
Anatomic Considerations
Most severe orofacial infections develop as a result of periapical, periodontal, or pericoronal dental infection (the latter surrounding the crown of an erupting tooth), with spread occurring along the anatomic pathways of least resistance. Generally, periodontal and pericoronal infections drain through the gingival sulcus into the oral cavity and rarely have major sequelae; the exception is pericoronal infections involving erupting third molar teeth, which can spread into deeper anatomic
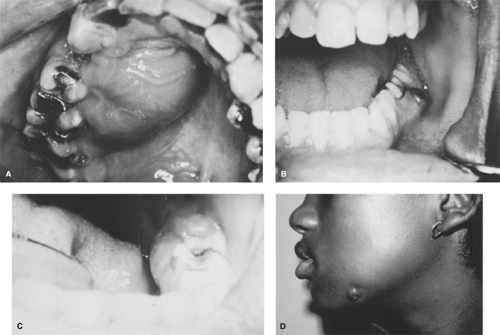
spaces because of their posterior location in the oral cavity. Conversely, usually infections associated with root apices are confined within the bony alveolar process. Should spontaneous intraoral drainage occur through either the periodontium or the pulp chamber, further spread through marrow spaces is unlikely. If such drainage does not occur, spread through bone (osteomyelitis) or perforation of the cortical plate of the affected jaw may take place. Once penetration of the cortical plate occurs, infection will involve the adjacent soft tissues and may manifest either as cellulitis or as a soft tissue abscess that eventually may perforate mucous membrane or skin as a sinus tract (Fig. 247.1).
spaces because of their posterior location in the oral cavity. Conversely, usually infections associated with root apices are confined within the bony alveolar process. Should spontaneous intraoral drainage occur through either the periodontium or the pulp chamber, further spread through marrow spaces is unlikely. If such drainage does not occur, spread through bone (osteomyelitis) or perforation of the cortical plate of the affected jaw may take place. Once penetration of the cortical plate occurs, infection will involve the adjacent soft tissues and may manifest either as cellulitis or as a soft tissue abscess that eventually may perforate mucous membrane or skin as a sinus tract (Fig. 247.1).
TABLE 247.1. MOST FREQUENT PATHOGENS ISOLATED IN OROFACIAL INFECTIONS IN TWO STUDIES | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
TABLE 247.2. TAXONOMIC CHANGES IN SELECTED ORAL PATHOGENS | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
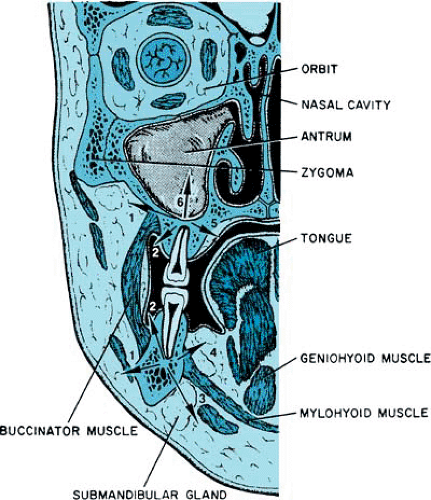
Usually, perforation of periapical infections through bone follows a typical pattern stemming from the position of the root apices in relation to the bony cortex and to muscle attachments (Fig. 247.2). Abscesses associated with anterior teeth and the buccal roots of maxillary posterior teeth tend to perforate labially or buccally (into the cheek) because the tooth roots are close to that cortical plate. Abscesses associated with mandibular posterior teeth may perforate either the buccal or the lingual cortical plate. When the spread of a mandibular infection occurs lingually, the relationship of the tooth apex to the mylohyoid muscle origin is significant. If the roots are superior to the mylohyoid muscle, infections will localize intraorally in the floor of the mouth (sublingual space). Usually, apices of the second and third molars are located inferior to
the mylohyoid muscle; consequently, the submandibular space will be involved, with extraoral localization.
the mylohyoid muscle; consequently, the submandibular space will be involved, with extraoral localization.
In children, often the maxillary and mandibular root apices are located superior and inferior, respectively, to the attachment of the buccinator muscle. Consequently, dental infections in younger patients may have a greater tendency to spread into the facial soft tissues and to localize extraorally.
Two fascial spaces commonly associated with odontogenic infections are the submandibular and masticator spaces. The submandibular space is formed by a splitting of the superficial layer of deep cervical fascia, superficial to the mylohyoid muscle and inferior to the mandible. Anteriorly and posteriorly, it is limited by the digastric muscle. Within this space lie the submandibular gland and portions of the facial artery and anterior facial vein. This space is approximated closely to the sublingual and lateral pharyngeal spaces. Often, infections of the submandibular space originate from lower molar teeth, and submandibular space infections either may spread to or arise from infections in the sublingual, lateral pharyngeal, or masticator spaces.
The masticator space also is formed by a splitting of the superficial layer of deep cervical fascia to surround the muscles of mastication and the lower jaw. Its contents include the masseter muscle, the internal and external pterygoid muscles, the temporal muscle, and the mandibular ascending ramus. The temporal and infratemporal spaces are the superior extensions of the masticator space. Adjacent are the submandibular, lateral pharyngeal, and retropharyngeal spaces. Infections of the masticator space may originate in adjacent spaces or can spread to it from periapical or pericoronal infections of the mandibular second and third molars and the maxillary third molar.
Diagnosis
Patients with odontogenic infections may have symptoms ranging from minor to life-threatening. Patients with an orofacial infection should receive thorough systemic and extraoral head and neck evaluations; importantly, the intraoral examination for a possible odontogenic cause must not be overlooked. Thorough intraoral evaluation begins with assessing the degree of mandibular opening. The interincisal distance on wide opening may be 40 mm or more, even in young children. Painful limitation of opening, or trismus, is associated with inflammation of the muscles of mastication and indicates spread of the infection to the masticator space. Teeth are inspected visually for caries, by percussion for tenderness, and by electric or hot and cold stimulation for pulpal involvement. Gingival tissues are probed for periodontal defects, and salivary glands are palpated for tenderness and are milked to observe for purulent discharge from the duct orifices.
Therapy
New treatment concepts for caries and periodontal disease, the most common oral infections, are evolving. These infections are coming to be viewed as a pathologic colonization of the oral cavity by specific normally nonresident pathogens. Traditional dental therapy for caries and periodontal disease aimed at suppressing the growth of a polymicrobial flora and repairing the damage that it causes. Instead, the goal of treatment would not be to control the growth of Streptococcus mutans (the primary agent of caries) or of a variety of periodontal pathogens by oral hygiene measures alone, but rather to eliminate S. mutans entirely.
Strategies for eradicating such organisms include the elimination of colonization sites for S. mutans by sealing the pits and fissures of the teeth with acrylic resins, use of such topical antiseptics as chlorhexidine, oral hygiene measures, and periodic culturing of saliva for this caries-producing organism. Similarly effective, especially in adults, may be elimination of periodontal pathogens by periodontal surgical débridement of the entire dentition at one visit and by a course of antibiotics effective against these organisms, followed by oral hygiene measures and topical fluoride therapy. Treatment protocols using these strategies are being tested. As with infections elsewhere in the body, the principles of treatment of orofacial infections involve surgical drainage and antibiotics. In a review of serious pediatric head and neck inpatient infections, Dodson and colleagues found that infections located above the upper lip originated most frequently in the sinuses or upper respiratory tract, whereas lower facial infections were primarily odontogenic. Almost always, odontogenic infections required surgical intervention, probably owing to the abscess-producing flora of odontogenic infections and the deep intrabony portal of entry afforded to those organisms by the roots of the teeth. Dental infections treated only with antibiotics almost always recur in a manifestation worse than the previous. Surgical drainage, preferably intraoral, may include standard incision and drainage of cellulitis or abscess or, in the case of localized periapical infection, endodontic drainage through the pulp (root canal therapy) or extraction of the offending tooth. Antibiotic therapy, although not necessary for minor, well-localized periapical lesions in noncompromised patients, is indicated if cellulitis or abscess, infections of adjacent bone (osteomyelitis), systemic signs (fever, dehydration, lymphadenopathy), trismus, or immunocompromise are present.
Generally, antibiotic selection for odontogenic infections, although based ultimately on Gram stain and aerobic and anaerobic cultures, is begun empirically before culture results
are available. Penicillin is the logical first choice for oral therapy of outpatient odontogenic infections because of its lack of toxicity, its bactericidal nature, and the sensitivity of most streptococci and oral anaerobes to this drug. Such aminopenicillins as ampicillin and amoxicillin with or without clavulanate have not demonstrated significantly better clinical success than that of penicillin V. If clinical signs or Gram stain results suggest the presence of Staphylococcus aureus, a penicillinase-resistant drug, such as oxacillin or dicloxacillin, may be added to the penicillin until the culture results are available. Alternately, a first- or second-generation cephalosporin, such as cephalexin or cefuroxime, may be used. In the case of penicillin allergy, clindamycin may be substituted, but it remains the drug of second choice for outpatient infections because of its greater toxicity. The macrolide antibiotics should be considered as tertiary selections for orofacial infections because of the high rate of resistance found among the oral streptococci and anaerobes. Among the macrolides, azithromycin would be the best choice, because of its ability to concentrate above serum levels in phagocytes. Tetracycline may result in severe staining of teeth in children younger than age 12 years and should be avoided, except when given specifically for Actinobacillus actinomycetemcomitans in cases of severe juvenile periodontitis. Similarly, the safety of using metronidazole in children has not been established, even though it is highly effective against obligate anaerobic bacteria. The fluoroquinolones are chondrotoxic in children, and their bacterial spectrum is not highly effective against most oral pathogens. Among the fluoroquinolones, however, moxifloxacin appears to have the optimum antimicrobial spectrum for odontogenic infections. However, orofacial infections severe enough to warrant hospital admission may differ microbiologically from less severe infections. Resistance to penicillin among the oral pathogens is increasing. In one study in the United Kingdom, 55% of the strains isolated were resistant to penicillin. Therefore, parenteral clindamycin, which remains highly effective against oral pathogens, is the antibiotic of first choice in severe odontogenic infections.
are available. Penicillin is the logical first choice for oral therapy of outpatient odontogenic infections because of its lack of toxicity, its bactericidal nature, and the sensitivity of most streptococci and oral anaerobes to this drug. Such aminopenicillins as ampicillin and amoxicillin with or without clavulanate have not demonstrated significantly better clinical success than that of penicillin V. If clinical signs or Gram stain results suggest the presence of Staphylococcus aureus, a penicillinase-resistant drug, such as oxacillin or dicloxacillin, may be added to the penicillin until the culture results are available. Alternately, a first- or second-generation cephalosporin, such as cephalexin or cefuroxime, may be used. In the case of penicillin allergy, clindamycin may be substituted, but it remains the drug of second choice for outpatient infections because of its greater toxicity. The macrolide antibiotics should be considered as tertiary selections for orofacial infections because of the high rate of resistance found among the oral streptococci and anaerobes. Among the macrolides, azithromycin would be the best choice, because of its ability to concentrate above serum levels in phagocytes. Tetracycline may result in severe staining of teeth in children younger than age 12 years and should be avoided, except when given specifically for Actinobacillus actinomycetemcomitans in cases of severe juvenile periodontitis. Similarly, the safety of using metronidazole in children has not been established, even though it is highly effective against obligate anaerobic bacteria. The fluoroquinolones are chondrotoxic in children, and their bacterial spectrum is not highly effective against most oral pathogens. Among the fluoroquinolones, however, moxifloxacin appears to have the optimum antimicrobial spectrum for odontogenic infections. However, orofacial infections severe enough to warrant hospital admission may differ microbiologically from less severe infections. Resistance to penicillin among the oral pathogens is increasing. In one study in the United Kingdom, 55% of the strains isolated were resistant to penicillin. Therefore, parenteral clindamycin, which remains highly effective against oral pathogens, is the antibiotic of first choice in severe odontogenic infections.
Types of Odontogenic Infections
Nursing-Bottle Caries
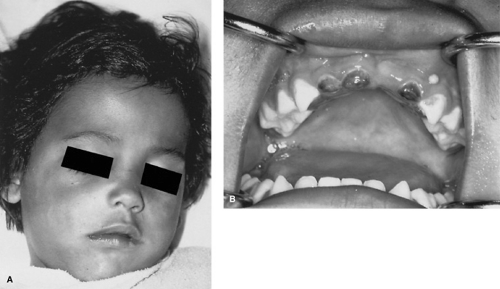
An identified syndrome of tooth decay affects primarily the primary upper incisors and frequently the upper and lower primary molars in children of bottle-feeding age (Fig. 247.3). It is caused by the practice of putting a child to bed with a nursing bottle filled with a sugar-containing drink, such as milk, fruit juices, or soft drinks. The child sucks on the bottle intermittently during sleep, when salivary secretion is low, and the sugar-containing liquids stay in the mouth for extended periods. This condition provides an excellent environment for the growth of caries-producing organisms. Nursing-bottle caries can destroy virtually the entire primary dentition of a child as it erupts. Therefore, pediatric physicians and dentists are advised to instruct parents to avoid putting children to bed with a nursing bottle or, if they must do so, to use only water in the bedtime drink.
Periapical Abscess
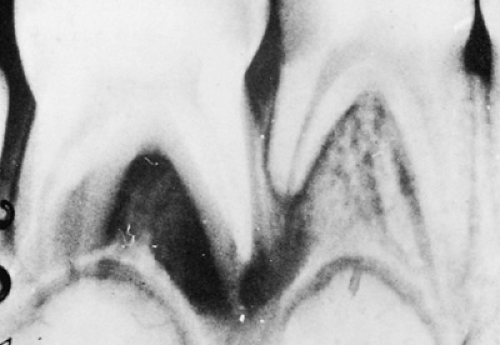
Extension of microorganisms through the root apex will lead to the formation of an abscess. Early in this process, the acute abscess is indistinguishable clinically and radiographically from acute pulpitis (toothache), particularly because radiographic evidence of bone destruction may take 21 days or more to develop. Sensitivity to heat stimuli (often relieved by cold), exquisite sensitivity to percussion, and tenderness to finger pressure on the alveolar process are indications that a tooth has become abscessed. Chronic abscesses are diagnosed more easily by observing looseness of the tooth, the presence of suppuration from draining sinuses or from the gingival crevice, and the presence of a periapical radiolucency on the radiograph (Fig. 247.4). Depending on the path of least anatomic resistance, tender swellings may be noted in the buccal or lingual mucosa. The classic presentation of swollen face or neck, pain, elevated
temperature, and malaise should direct clinicians to suspect an odontogenic infection. Adequate surgical drainage is the key principle in the treatment of periapical abscesses and may be accomplished by endodontic (root canal) therapy, extraction of the tooth, or incision and drainage, as necessary. Antibiotic therapy should be considered an adjunctive rather than a primary therapeutic modality, because antibiotics alone will not remove the cause of the infection.
temperature, and malaise should direct clinicians to suspect an odontogenic infection. Adequate surgical drainage is the key principle in the treatment of periapical abscesses and may be accomplished by endodontic (root canal) therapy, extraction of the tooth, or incision and drainage, as necessary. Antibiotic therapy should be considered an adjunctive rather than a primary therapeutic modality, because antibiotics alone will not remove the cause of the infection.
Extraction of unsalvageable abscessed teeth soon after the diagnosis has been made has been found to be curative in approximately 96% of cases. Early extraction has also been shown to be associated with a decreased requirement for extraoral incision and drainage procedures, as compared with treatment only with antibiotics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree