There is no current cure for Duchenne muscular dystrophy (DMD), and palliative and prophylactic interventions to improve the quality of life of patients remain limited, with the exception of corticosteroids. This article describes 2 potential nutritional interventions for the treatment of DMD, green tea extract (GTE) and the branched-chain amino acid leucine, and their positive effects on physical activity. Both GTE and leucine are suitable for human consumption, are easily tolerated with no side effects, and, with appropriate preclinical data, could be brought forward to clinical trials rapidly.
Duchenne muscular dystrophy (DMD) is a lethal, X-linked recessive, muscle-wasting disease caused by mutations in the dystrophin gene, located on chromosome Xp21. Mutations of the dystrophin gene result in the absence of the dystrophin protein, which leads to an impaired linkage between the F-actin cytoskeleton and the extracellular matrix protein laminin 2 via the membrane-bound dystrophin-glycoprotein complex (DGC).
In the absence of dystrophin, the mechanical links from the cytoskeleton of the muscle cell to the membrane and the components of the DGC are absent. Progressive and ultimately fatal rounds of skeletal muscle degeneration and regeneration are hypothesized to result from either a fragile or weakened skeletal muscle membrane or altered cell signaling.
Beyond these general hypotheses, the specific cellular mechanisms and the temporal progression of the dystrophic process are as yet unclear. There is no current cure for DMD, and palliative and prophylactic interventions to improve the quality of life of patients remain limited, with the exception of corticosteroids. Corticosteroids are effective at prolonging ambulation but have several undesirable side effects, including growth retardation, obesity, glucose intolerance, and bone demineralization. Nevertheless, despite these side effects, a recent panel of experts recommended glucocorticoid therapy for all patients who have DMD. This recommendation suggests that until a suitable corticosteroid substitute is available, any additional palliative and prophylactic treatment approaches will likely be in conjunction with corticosteroids.
This article describes two potential nutritional interventions for the treatment of DMD, green tea extract (GTE) and the branched-chain amino acid (BCAA) leucine, and their positive effects on physical activity. Both GTE and leucine are suitable for human consumption; are easily tolerated with no side effects; and, with appropriate preclinical data, could be brought forward to clinical trials rapidly. In dystrophic mdx mice, both GTE and leucine (Voelker KA, unpublished data, 2010) improve whole animal endurance and skeletal muscle function. Mechanistically both are mediated by signaling pathways to evoke these and other positive adaptations that attenuate the effects of dystrophic progression. To date, not all the specific pathways have been described.
Characteristics of DMD
The characteristics of DMD have been well described at the genetic, molecular, cellular, tissue, organ systems, and clinical levels ( Table 1 ). Detailed descriptions are provided in several excellent reviews.
| Level of Pathology | Definitions and Descriptors at Various Levels of the Disease |
|---|---|
| Genetic | X-linked, hereditary or spontaneous |
| Cellular | Absence of the protein dystrophin, mechanical weakening of the sarcolemma, inappropriate calcium influx, recurrent muscle ischemia, aberrant cell signaling, increased oxidative stress, histologic z-disk disruption, central nucleation, fiber size heterogeneity, reduced expression and mislocalization of dystrophin-associated proteins |
| Tissue | Intramuscular accumulation of fibrous connective and fatty tissue, pseudohypertrophy |
| Organ systems | Musculoskeletal system, nervous system, digestive system, immune system, cardiorespiratory system |
| Clinical | Lethal, progressive, 1:3500 live male births, muscle wasting and weakness, susceptibility to fatigue, muscle pain, elevated serum creatine kinase, myoglobinuria, Gower sign, lordosis, progressive difficulty with ambulation, contractures, contraction-induced injury, secondary disuse atrophy, increased fat mass, side effects of medications, cardiorespiratory failure |
Best Practices of Care
DMD is a complex disease to manage. Bushby and colleagues recently published a detailed set of recommendations for the management of DMD. Among the many recommendations are those related to nutrition and exercise (physical activity). It is not the authors’ intent here to discuss all the difficulties associated with nutrition (eg, swallowing problems) or exercise (eg, spinal deformities) but to focus on simple nutritional possibilities that may attenuate disease severity and progression.
Importance of Mobility
A goal for treatment of patients with DMD should be to improve quality of life, one important aspect of which is mobility. Mobility is dependent on sufficient strength and endurance in skeletal muscles to move joints through a range of motion to accomplish a movement task. Some tasks may be occasional movements significant in everyday life, such as reaching for a glass. Other movements may be repetitive and rhythmic, such as walking. Because ambulatory muscles, the diaphragm, and the heart are all adversely affected by dystrophin deficiency, mobility in individuals with DMD is severely compromised. Can nutritional therapies improve mobility?
Why nutritional and physical activity therapies?
The US government has established guidelines for a balanced diet to meet the energy demands and macronutrient and micronutrient requirements for health ( http://health.gov/dietaryguidelines/2010.asp ), which includes balancing calories with physical activity to manage weight. Similarly, guidelines have been established by the Centers for Disease Control and Prevention for a minimum participation in physical activity on a daily basis ( http://www.cdc.gov/physicalactivity/everyone/guidelines/index.html ). At the most basic level, nutrition represents energy intake and adequate vitamins and minerals; physical activity represents energy output. These requirements are no less, and likely more, important for individuals with DMD.
Why nutritional and physical activity therapies?
The US government has established guidelines for a balanced diet to meet the energy demands and macronutrient and micronutrient requirements for health ( http://health.gov/dietaryguidelines/2010.asp ), which includes balancing calories with physical activity to manage weight. Similarly, guidelines have been established by the Centers for Disease Control and Prevention for a minimum participation in physical activity on a daily basis ( http://www.cdc.gov/physicalactivity/everyone/guidelines/index.html ). At the most basic level, nutrition represents energy intake and adequate vitamins and minerals; physical activity represents energy output. These requirements are no less, and likely more, important for individuals with DMD.
What is currently known
There has been little research published on effective nutrition or physical activity for individuals with DMD. Although it is recognized that genetic and molecular biological approaches will ultimately reveal a cure for DMD, is it prudent to overlook potential simple approaches, such as diet and physical activity, as palliative and prophylactic treatments until the cure is found? Unfortunately, these treatments are simply not being investigated rigorously. Simply put, little is known about the nutritional needs of patients with DMD and little is known about the potential positive (or negative) adaptive responses of dystrophic muscles to physical activity.
Nutrition
Davidson and Truby reported in their review that of approximately 1500 references they found on DMD, only 6 directly investigated the nutritional requirements of boys with DMD. Bushby and colleagues cited a similar small number of references, although some differed from those cited by Davidson and Truby. The total number of nutritional investigations seems to be only about 10 to 12. Based on these studies, the recommendations for nutritional guidance could be dramatically improved.
Physical Activity
The effects of physical activity to treat DMD have been investigated for several decades (eg, Refs. ), yet there are still no defined exercise prescriptions that include intensity, duration, and frequency. A recent review by Markert and colleagues suggested that appropriately prescribed physical activity might counter key dystrophic pathogenic mechanisms, including (1) mechanical weakening of the sarcolemma, (2) inappropriate calcium influx, (3) aberrant cell signaling, (4) increased oxidative stress, and (5) recurrent muscle ischemia.
Energy Balance
Although this article focuses on two specific nutritional interventions, monitoring the energy content of diet is also a cornerstone of health and is especially important to consider in disease states such as DMD, which affect multiple organ systems. Excessive caloric intake leads to conditions of overweight or obesity, whereas inadequate caloric intake precedes weight loss. Boys treated with steroids gain nonfunctional mass (eg, fat, not muscle) because appetite is stimulated. In boys with DMD whose mobility is compromised, this problem is exacerbated because they take in more energy but expend less.
Energy IN is determined by the amount consumed and the content of the diet in kilocalories. Energy OUT includes the sum of the resting metabolic rate, the thermic effect of food, the nonexercise energy expenditure, and exercise energy cost, also measured in kilocalories. In addition, the disease and medications can contribute to energy status in patients with DMD. When energy IN exceeds energy OUT, weight gain results. Systematic studies of energy expenditure in patients with DMD, using metabolic equivalents (MET = 3.5 mL oxygen/kg body mass/min), have been suggested to better prescribe physical activity and exercise guidelines. These studies would also help inform dietary guidelines for energy intake.
Pharmaceutical and Nutritional Interventions
Although the main impetuses to cure DMD are focused on genetic and molecular biological approaches, nutritional therapies could represent an appropriate and simple palliative approach. For example, treatment with the amino acids glutamine and arginine plus deflazacort were reported to improve nitrogen retention and maintain protein balance in patients with DMD. However, at present there have been few detailed investigations of nutritional interventions in DMD. Radley and colleagues succinctly provided a summary and analysis of several potential pharmaceutical and nutritional interventions as therapeutic agents in mdx mice and DMD. Among the potential nutritional interventions cited was GTE, which the investigators suggested was ready for clinical trial with the exception of the appropriate dose. In addition, several amino acids were also cited for possible use in treating DMD including taurine, glutamine, alanine, and arginine (alone or in various combinations). However, a caveat to the use of these amino acids was that they had yet to be formally evaluated. The potential benefits of leucine were not reported. The authors now summarize briefly the current literature on GTE, provide the likely signaling pathways of leucine to induce protein synthesis, and provide evidence of the benefits of leucine on strength in mdx mice and whole-body endurance.
GTE
GTE has been reported to ameliorate dystrophic pathology in mdx mice. Initial studies indicated that oxidative stress may contribute to muscular dystrophy symptoms, and early administration of dietary GTE to young mdx mice (and their dams, before weaning) protected against muscle necrosis in the extensor digitorum longus (EDL) muscle. Recognized for its antioxidative properties, GTE was investigated further as a possible protection against progression of muscular dystrophy. Administration early in the course of the disease was repeated in a study that compared GTE with its major component, epigallocatechin gallate (ECGC), a polyphenol. This study also showed reduced necrosis in muscles from GTE-treated and ECGC-treated mice, and furthermore reported improved muscle strength and fatigue resistance in functional assays.
In another study, voluntary exercise (wheel running) and GTE were investigated in 21-day-old mdx mice. Both conditions independently showed beneficial effects in assays of contractile properties, metabolic activity, lipid peroxidation, and antioxidant capacity. Synergistic effects of the combined treatments were also reported to benefit endurance capacity, although some other beneficial effects of GTE were mitigated by running. Mechanistic and time-course data indicate that GTE potentially ameliorates pathology by acting on the nuclear factor κB pathway. Histologic assays of GTE-treated mdx muscle showed a reduced area of regenerating fibers, indicating a protective effect, and a fiber morphology more like that of nondiseased muscle. In summary, GTE and its polyphenolic constituents merit further study as possible regulators of oxidative stress and inflammation. Just as light swimming exercise may benefit aerobic and cardiorespiratory capacity, particularly of young ambulatory patients with DMD, nutraceuticals such as BCAAs and GTE may provide benefit in additional biochemical and functional outcome measures ( Fig. 1 ).
Leucine
Leucine is an essential BCAA with unique features. In addition to being a building block of proteins, it is an anabolic signal that induces protein synthesis. In addition, it plays a role in glucose homeostasis. Leucine also acts as a nitrogen donor for the synthesis of alanine and glutamine in the muscle. Glutamate, which itself is the precursor of γ-aminobutyric acid (GABA), is produced from the transamination reaction of leucine and other BCAAs, and is a major excitatory neurotransmitter.
Leucine also improves nitrogen retention by increasing muscle protein synthesis and decreasing myofibrillar breakdown in normal pigs. Although both of these leucine-related effects would be relevant to reversal of the degradation processes in dystrophic muscle, it is not known whether dystrophic muscle will respond similarly. There is only one controlled clinical trial (conducted in 1984) that has investigated the therapeutic potential of leucine. This study demonstrated a transient increase in muscle strength reported after the first month of a 12-month trial, but results were later confounded by the unusual rate of functional decline in the placebo group. More recently, D’Antona and colleagues reported that supplementation with BCAAs promoted longevity as well as skeletal and cardiac muscle biogenesis in middle-aged mice, including enhanced physical endurance. Similarly, the authors’ recent preliminary data demonstrating improved contractile and endurance performance in the mdx mouse indicate that leucine may be an effective nutritional therapy for DMD.
Mammalian target of rapamycin
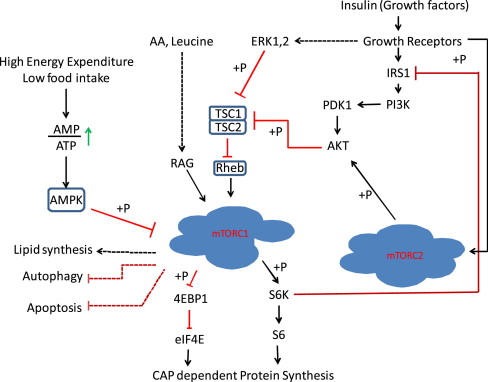
Although it is well established that leucine can stimulate protein synthesis through the mammalian target of rapamycin (mTOR) signaling pathway, the identity of sensor(s) for leucine is not known. The strongest link between amino acids and mTOR complex 1 (mTORC1) is the Rag guanosine triphosphatases, which, in response to amino acids, bind to raptor ( Fig. 2 ). This interaction alters the intracellular location of the mTORC1 to a compartment where its activator Rheb is present. Activated mTORC1 phosphorylates substrates, of which ribosomal S6 protein kinase (S6K) and 4E-binding protein-1 (4EBP-1) are well known.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








