Although prednisone has never been formally approved for use in Duchenne muscular dystrophy (DMD) by regulatory agencies, its efficacy has been confirmed in trials dating from the 1980s. There is a strong need for optimization of both specific type of glucocorticoid (eg, prednisone, vs deflazacort or others) and the dosing regimen. Ideally an optimized regimen would maximize efficacy while minimizing side-effect profiles. A new trial, FOR-DMD, aims to address this gap in knowledge. In parallel, there has been progress in the area of “dissociative steroids,” drugs that are able to better separate efficacy and side effects, providing a broader therapeutic window.
- •
Current standard of care of Duchenne muscular dystrophy (DMD) includes pharmacologic treatment with oral glucocorticoids.
- •
Gains in strength and slowed progression of disease afforded by glucocorticoids are offset, in part, by the wide range of side effects of drug treatment.
- •
Dose optimization studies are limited, and new larger clinical studies are needed to best balance efficacy and side effects (therapeutic window), as are studies of glucocorticoid alternatives to prednisone.
- •
The FOR-DMD trial funded by the National Institutes of Health is under way to compare different dose regimens and types of glucocorticoids (prednisone, deflazacort).
- •
A novel dissociative steroid, a Δ-9,11 drug, is under clinical development for DMD. This drug promises to broaden the therapeutic window and reduce side-effect profiles.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked progressive muscular dystrophy, caused by loss of the dystrophin protein at the myofiber membrane. Pharmacologic treatment of DMD patients with glucocorticoids can improve patient strength and prolong ambulation, with concomitant improvements in quality-of-life scales. As such, glucocorticoid treatment for DMD is recommended in standard-of-care guidelines, and as an American Academy of Neurology practice parameter. The majority of trials and treatment recommendations have used an oral dose of prednisone at 0.75 mg/kg/d. However, alternative dosing regimens have been reported as changing the efficacy versus side-effect profiles, including weekend dosing, lower doses, and alternative-day doses (10 mg/kg/wk divided over 2 weekend days). In each study, a goal was to achieve a better balance of efficacy (increased strength and delay of disease progression) with fewer side effects (bone fragility, weight gain, mood changes). It is pertinent to note that muscle weakness and wasting is an acknowledged side effect of chronic glucocorticoid administration in many indications, such as critical care medicine, and is the most common drug-induced form of muscle weakness. Glucocorticoids have a direct molecular effect on myofibers, stimulating the catabolic AKT1/FOXO1 pathway, decreasing protein synthesis and increasing the rate of protein catabolism, resulting in weakness and atrophy. Thus it is likely that DMD patients treated with glucocorticoids show the clinical outcome of increased muscle strength mitigated to some extent by the side effect of muscle catabolism. Clearly any effort to reduce side effects such as weight gain and short stature may also lead to lessening of the side effect of muscle weakness, whereby the balance would then be tipped to greater efficacy.
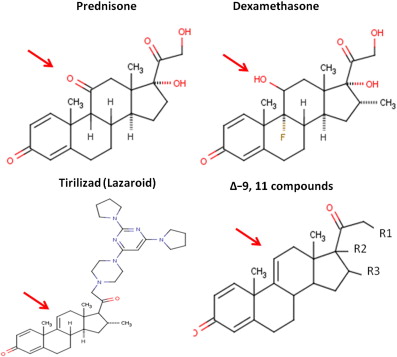
Fluorinated glucocorticoids, such as dexamethasone, are considerably more potent, with higher affinity to the glucocorticoid receptor ( Fig. 1 ). However, these tend to also exacerbate side effects, and are generally avoided in indications of chronic use, such as muscular dystrophy. On the other hand, less potent nonfluorinated varieties of glucocorticoids have been tried, such as deflazacort. Deflazacort trials in DMD have suggested similar efficacy to that of prednisone (albeit at a higher dose), with an improvement in some side-effect profiles.
Finding the optimum regimen of corticosteroids for DMD (FOR-DMD) clinical trial
To study the balance of efficacy and side effects, depending on steroid type (prednisone vs deflazcort) and dosing regimen (daily vs 10 days on, 10 days off), the FOR-DMD trial was designed and implemented. FOR-DMD is a multicenter, double-blind, parallel-group, 36- to 60-month study, comparing 3 corticosteroid regimens in wide use in DMD:
- •
Daily prednisone (0.75 mg/kg/d)
- •
Intermittent prednisone (0.75 mg/kg/d, 10 days on, 10 days off)
- •
Daily deflazacort (0.9 mg/kg/d).
The hypothesis being tested is that daily corticosteroids (prednisone or deflazacort) will be of greater benefit than intermittent corticosteroids (prednisone) in terms of function and subject/parent satisfaction. A secondary outcome is to study whether daily deflazacort will be associated with a better side-effect profile than daily prednisone.
The primary outcome variable will be a 3-dimensional (multivariate) outcome consisting of the following 3 components (each averaged over all postbaseline follow-up visits through month 36): (1) time to stand from lying (log-transformed), (2) forced vital capacity, and (3) subject/parent global satisfaction with treatment, as measured by the Treatment Satisfaction Questionnaire for medication.
Secondary outcome variables will include regimen tolerance, adverse event profile, and secondary functional outcomes including the 6-minute walk test, quality of life, and cardiac function. The analyses will be adjusted for covariates, namely country/region, baseline time to stand from lying, baseline forced vital capacity (FVC), and initial weight band. A sample size of 100 subjects per group (300 in total) will provide adequate power to detect differences that are thought to be of minimal clinical significance between any 2 of the 3 treatment groups, assuming a 10% rate of subject withdrawal.
The trial will randomize 300 boys aged 4 to 7 years to 0.75 mg/kg/d prednisone; 0.75 mg/kg/d prednisone for 10 days alternating with 10 days off; or 0.9 mg/kg/d deflazacort. All boys will complete a minimum 3 years (36 months) treatment period. All boys entering the trial will remain on the study drug until the last boy completes the 36 months of study; this may be up to 60 months.
Eligible boys will be those with confirmed DMD (defined as male with clinical signs compatible with DMD and confirmed DMD mutation in the dystrophin gene [out-of-frame deletion or point mutation or duplication] or absent/<3% dystrophin on muscle biopsy); age at least 4 years and under 8 years; ability to rise independently from the floor; willingness and ability of parent or legal guardian to give informed consent; willingness and ability to comply with scheduled visits, drug administration plan, and study procedures; and ability to maintain reproducible FVC measurements.
The study is funded by the National Institutes of Health (Kate Bushby and Robert Griggs, study Chairs), and will begin enrollment in 2012.
Finding the optimum regimen of corticosteroids for DMD (FOR-DMD) clinical trial
To study the balance of efficacy and side effects, depending on steroid type (prednisone vs deflazcort) and dosing regimen (daily vs 10 days on, 10 days off), the FOR-DMD trial was designed and implemented. FOR-DMD is a multicenter, double-blind, parallel-group, 36- to 60-month study, comparing 3 corticosteroid regimens in wide use in DMD:
- •
Daily prednisone (0.75 mg/kg/d)
- •
Intermittent prednisone (0.75 mg/kg/d, 10 days on, 10 days off)
- •
Daily deflazacort (0.9 mg/kg/d).
The hypothesis being tested is that daily corticosteroids (prednisone or deflazacort) will be of greater benefit than intermittent corticosteroids (prednisone) in terms of function and subject/parent satisfaction. A secondary outcome is to study whether daily deflazacort will be associated with a better side-effect profile than daily prednisone.
The primary outcome variable will be a 3-dimensional (multivariate) outcome consisting of the following 3 components (each averaged over all postbaseline follow-up visits through month 36): (1) time to stand from lying (log-transformed), (2) forced vital capacity, and (3) subject/parent global satisfaction with treatment, as measured by the Treatment Satisfaction Questionnaire for medication.
Secondary outcome variables will include regimen tolerance, adverse event profile, and secondary functional outcomes including the 6-minute walk test, quality of life, and cardiac function. The analyses will be adjusted for covariates, namely country/region, baseline time to stand from lying, baseline forced vital capacity (FVC), and initial weight band. A sample size of 100 subjects per group (300 in total) will provide adequate power to detect differences that are thought to be of minimal clinical significance between any 2 of the 3 treatment groups, assuming a 10% rate of subject withdrawal.
The trial will randomize 300 boys aged 4 to 7 years to 0.75 mg/kg/d prednisone; 0.75 mg/kg/d prednisone for 10 days alternating with 10 days off; or 0.9 mg/kg/d deflazacort. All boys will complete a minimum 3 years (36 months) treatment period. All boys entering the trial will remain on the study drug until the last boy completes the 36 months of study; this may be up to 60 months.
Eligible boys will be those with confirmed DMD (defined as male with clinical signs compatible with DMD and confirmed DMD mutation in the dystrophin gene [out-of-frame deletion or point mutation or duplication] or absent/<3% dystrophin on muscle biopsy); age at least 4 years and under 8 years; ability to rise independently from the floor; willingness and ability of parent or legal guardian to give informed consent; willingness and ability to comply with scheduled visits, drug administration plan, and study procedures; and ability to maintain reproducible FVC measurements.
The study is funded by the National Institutes of Health (Kate Bushby and Robert Griggs, study Chairs), and will begin enrollment in 2012.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








