Surgical management of spinal deformity in neuromuscular diseases (NMDs) often requires a multidisciplinary approach beginning in the preoperative surgical planning period, owing to concomitant restrictive lung disease and cardiomyopathy in selected NMD conditions. The need for thorough and thoughtful discussions must occur with the family and other caregivers before any scheduled surgery. The decision to proceed with spinal instrumentation may alter functional abilities in weak and marginally ambulatory NMD patients. With care and treatment involving a multidisciplinary team, proper planning, and support, patients will likely experience rewarding outcomes and improved quality of life.
- •
Spinal deformity adversely affects the quality of life of patients with progressive hereditary neuromuscular diseases (NMDs).
- •
While the impact of spinal arthrodesis on pulmonary function remains controversial (the disease pathogenesis continues to affect chest wall muscles and diaphragm), there can be no doubt that failure to aggressively treat spinal deformity in progressive NMD can have profound negative consequences on sitting balance, pelvic obliquity, ability to sit comfortably in a wheelchair, cosmesis, and quality of life.
- •
There are emerging data that improvements in disease management in Duchenne muscular dystrophy (DMD) including treatment with corticosteroids, surgical management of spine deformity, noninvasive ventilation, and more effective treatment of cardiomyopathy have led to improved function and survival in DMD and a changing natural history of disease.
- •
Spinal arthrodesis with internal instrumentation is the only effective treatment, but optimally is deferred to the second decade so that anterior approaches are avoided. Spinal orthotics may be used in younger patients to provide postural support and more balanced sitting, but spinal orthotics generally do not affect the natural history of spinal deformity in NMD conditions.
- •
Surgical management of the spinal deformity often requires a multidisciplinary approach beginning in the preoperative surgical planning period owing to concomitant restrictive lung disease and cardiomyopathy in selected NMD conditions.
Introduction
The treatment of pediatric scoliosis and kyphosis falls under 3 categories: idiopathic, congenital, and neuromuscular. The focus of this article is on neuromuscular spine deformity occurring in children with neuromuscular diseases (NMDs). In this context, NMDs are specifically defined by defects in function of the anterior horn cell (motor neuron diseases), the peripheral nerve system (neuropathies), the neuromuscular junction (congenital myasthenia), or muscles (eg, muscular dystrophies). For the spine surgeon, disease processes such as those that involve the central nervous system, such as cerebral palsy or spina bifida, are often combined into the umbrella term of neuromuscular scoliosis. However, this review focuses on pediatric diseases of the lower motor neuron (ie, NMDs) that are associated with spinal deformity.
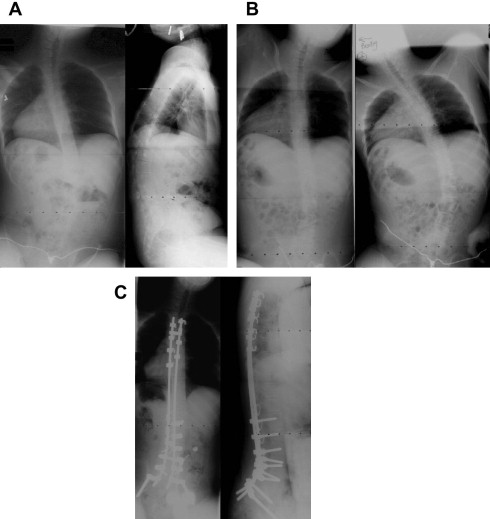
Severe spinal deformity in progressive NMD leads to multiple problems, including poor sitting balance, difficulty with upright seating and positioning, pain, difficulty in attendant care, and potential exacerbation of underlying restrictive respiratory compromise. Severe scoliosis and pelvic obliquity can in some instances completely preclude upright sitting in a wheelchair. Aggressive management of spinal deformity with bracing and spinal instrumentation have been based on the assumption that progressive spinal deformity leads to poor sitting balance, pelvic obliquity, and greater likelihood of pressure sores, functional impairment, and poor cosmesis, as well as reduced quality of life. While the impact of spinal arthrodesis on pulmonary function remains controversial (the disease pathogenesis continues to affect chest wall muscles and diaphragm), there can be no doubt that failure to aggressively treat spinal deformity in progressive NMD can have profound negative consequences on sitting balance, pelvic obliquity, ability to sit comfortably in a wheelchair, cosmesis, and quality of life ( Fig. 1 A). In addition, there are emerging data that improvements in disease management in Duchenne muscular dystrophy (DMD) including treatment with corticosteroids, surgical management of spine deformity, noninvasive ventilation, and more effective treatment of cardiomyopathy have led to improved function and survival in DMD and a changing disease natural history.
Prevalence and natural history of spinal deformity in NMDs
Spinal deformity is a well-documented sequela of childhood-onset NMD and in some adults with NMD. The reported ultimate prevalence of scoliosis in DMD previously varied from 33% to 100% in the literature. McDonald and colleagues showed that 50% of males with DMD acquire scoliosis between 12 and 15 years of age, corresponding to the adolescent growth spurt. This study found that 10% of older DMD subjects showed no clinical scoliosis. This finding is consistent with that of Oda and colleagues of a 15% prevalence of DMD patients with mild nonprogressive curves (usually 10°–30°). The rate of progression of the primary or single untreated curve has been reported to range from 11° to 42° per year, depending on the age span studied. Oda and colleagues have shown that curve severity in the sagittal plane was a predictor of curve progression as well as pulmonary function changes over time. Most patients who developed progressive scoliosis showed less than 2000 mL peak obtained forced vital capacity (FVC). More recently, the incidence of scoliosis in DMD has been shown to be significantly reduced in those patients treated with glucocorticoids.
Scoliosis has been estimated to occur in 78% to nearly 100% of patients with spinal muscular atrophy (SMA) type II. Severity of weakness and age (skeletal maturity) have been described as critical factors influencing the risk for SMA patients of developing spinal deformity. Scoliosis almost always begins in the first decade of life as a result of sever truncal weakness. The curves are collapsing in nature, and may be thoracolumbar, thoracic, lumbar, double curves involving the thoracolumbar and thoracic regions, or double curves involving the thoracic and thoracolumbar regions. In SMA, the average deformity is 90°, with a reported range from 20° to 164°. Severe kyphosis may be a common associated deformity, and almost all persons with severe scoliosis have significant pelvic obliquity. By contrast, patients with SMA type III who are still ambulating show a lower incidence of scoliosis, reported to be 8% to 63%.
In addition, spinal deformity has been shown by the authors’ group to be common in severe childhood autosomal recessive limb-girdle muscular dystrophy (LGMD), congenital muscular dystrophy, and congenital myotonic muscular dystrophy. Adults with facioscapulohumeral muscular dystrophy (FSHD) may develop collapsing hyperlordosis. In 2 previous studies of the natural history of scoliosis in Friedreich ataxia, the prevalence of scoliosis approached 100%.
Prevalence and natural history of spinal deformity in NMDs
Spinal deformity is a well-documented sequela of childhood-onset NMD and in some adults with NMD. The reported ultimate prevalence of scoliosis in DMD previously varied from 33% to 100% in the literature. McDonald and colleagues showed that 50% of males with DMD acquire scoliosis between 12 and 15 years of age, corresponding to the adolescent growth spurt. This study found that 10% of older DMD subjects showed no clinical scoliosis. This finding is consistent with that of Oda and colleagues of a 15% prevalence of DMD patients with mild nonprogressive curves (usually 10°–30°). The rate of progression of the primary or single untreated curve has been reported to range from 11° to 42° per year, depending on the age span studied. Oda and colleagues have shown that curve severity in the sagittal plane was a predictor of curve progression as well as pulmonary function changes over time. Most patients who developed progressive scoliosis showed less than 2000 mL peak obtained forced vital capacity (FVC). More recently, the incidence of scoliosis in DMD has been shown to be significantly reduced in those patients treated with glucocorticoids.
Scoliosis has been estimated to occur in 78% to nearly 100% of patients with spinal muscular atrophy (SMA) type II. Severity of weakness and age (skeletal maturity) have been described as critical factors influencing the risk for SMA patients of developing spinal deformity. Scoliosis almost always begins in the first decade of life as a result of sever truncal weakness. The curves are collapsing in nature, and may be thoracolumbar, thoracic, lumbar, double curves involving the thoracolumbar and thoracic regions, or double curves involving the thoracic and thoracolumbar regions. In SMA, the average deformity is 90°, with a reported range from 20° to 164°. Severe kyphosis may be a common associated deformity, and almost all persons with severe scoliosis have significant pelvic obliquity. By contrast, patients with SMA type III who are still ambulating show a lower incidence of scoliosis, reported to be 8% to 63%.
In addition, spinal deformity has been shown by the authors’ group to be common in severe childhood autosomal recessive limb-girdle muscular dystrophy (LGMD), congenital muscular dystrophy, and congenital myotonic muscular dystrophy. Adults with facioscapulohumeral muscular dystrophy (FSHD) may develop collapsing hyperlordosis. In 2 previous studies of the natural history of scoliosis in Friedreich ataxia, the prevalence of scoliosis approached 100%.
Clinical management of neuromuscular scoliosis
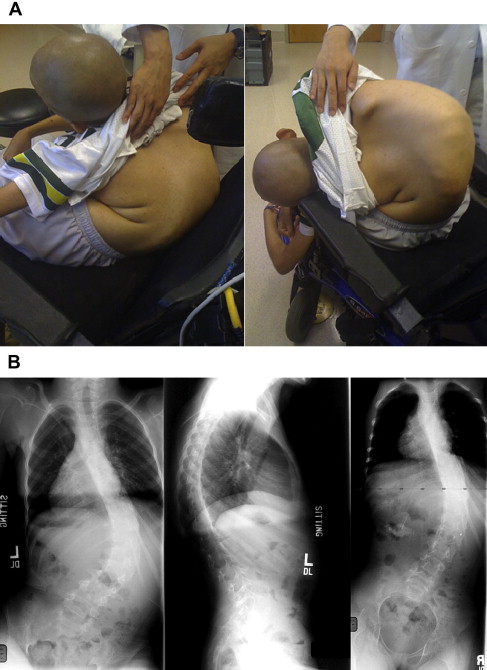
The early childhood period (age <8 years) is best managed with upright seating support allowing semirecumbent posture with lateral uprights to allow control of coronal plane collapse, and a wheelchair cushion providing weight transfer to the greatest surface area of the thigh. Early in the disease course of patients with scoliosis related to hypotonia, spinal curvatures are flexible and will completely correct with supine positioning. By contrast, upright radiographs (seated) will demonstrate global spinal collapse in the coronal plane and either kyphotic or lordotic collapse on the sagittal plane (lateral radiograph), which may depend on disease process or stage of disease. For children with spontaneous curve correction on supine radiographs, further observation and seating-support management is desirable. Young children with hypotonia and postural spine deformity may benefit from provision of a soft spinal orthosis to help with sitting balance.
For DMD patients the authors often obtain anteroposterior (AP) and lateral scoliosis films every 6 months between the ages of 11 and 16 for DMD, or earlier if they progress to full-time reliance on the wheelchair at a younger age. Curve progression can be unpredictable in this age group, and appropriate clinical surveillance is critical. It is not uncommon in this patient population to obtain regular pulmonary function tests (particularly for DMD) at least once if not twice a year during this critical age range to establish the peak FVC. Oda and colleagues described an association between increased peak FVC and decreased curve progression in DMD patients. McDonald and colleagues described DMD patients with peak FVC greater than 2.5 L/min to have milder disease progression than those with peak FVC less than 1.7 L/min. Therefore, use of peak FVC may act as a prognostic indicator for severity of spinal deformity in DMD. Radiographic surveillance begins earlier in patients with SMA II, owing to the earlier onset of spine deformity.
Spinal Bracing for Management of Spine Deformity in NMD
The use of orthosis in the management of spinal deformity has been studied extensively. The use of orthotics is generally poorly tolerated by the neuromuscular patient population, and may create a situation whereby the FVC can be lowered with these devices unless care is taken to provide an anterior soft opening to accommodate chest-wall expansion and abdominal protuberance caused by predominant diaphragm breathing patterns. Bracing has been proved to be ineffective, with no clear evidence in the literature that orthotics prevent progression of spinal deformity in the NMD patient population. If bracing is prescribed in a patient with NMD, the indication is usually the need for a soft thoraco-lumbo-sacral orthosis to help with sitting balance. In SMA type II, prophylactic bracing has been used by many clinicians to delay the development of spinal deformity to the second decade and/or decrease the severity of deformity so that the child can grow as much as possible before spinal fusion. Even in patients with adolescent idiopathic scoliosis, bracing continues to be controversial. For example, Negrini and colleagues, in a Cochrane review of bracing in adolescent idiopathic scoliosis, were unable to draw definitive conclusions regarding bracing based on the lack of data from randomized control studies that used the Scoliosis Research Society (SRS) guidelines for study. In other words, a meta-analysis could not be performed for lack of appropriate level of evidence in the literature. The authors’ clinical experience shows that bracing can improve younger NMD patient’s sitting posture once curve progression has occurred, while being are aware that it may not obviate surgical intervention.
In a majority of neuromuscular patients with scoliosis, the lumbar and thoracolumbar spine serves as the apex or focus of spinal deformity, with a resultant truncal decompensation. Impaired sitting balance also occurs related to pelvic obliquity, which when present is frequently rigid and not well compensated for by alterations in seating (custom or compensated seat cushioning). In these children and adolescents, excessive tilt is noted by parents and caregivers. More commonly the coronal plane deformity is accompanied by a kyphotic sagittal plane alignment that also interferes with upright sitting posture. It is relatively common for patients and caregivers to relate back-pain complaints as scoliosis worsens. Previous guidelines in neuromuscular patients recommended surgical treatment for scoliosis once angular deformity exceeded 25°, citing an optimal match between Cobb angle as predictive of relentless progression with favorable pulmonary volumes.
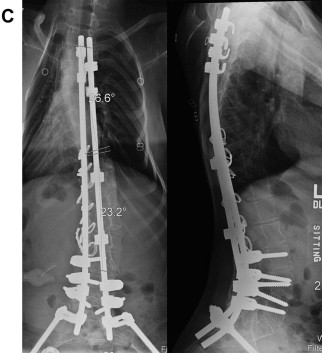
Because of recent advances in pediatric critical care management and postoperative respiratory therapy support, surgical treatment of spine deformity in DMD can be deferred until Cobb angle measurements approach 45° to 50° and may be safely tolerated in patients with FVC measurements even below 30% to 40% predicted. In the authors’ recently published series on DMD, operative intervention was performed on patients of average age approximately 12 years with average curve size of 47°. The greatest curve seen in this series, however, measured more than 100°, reflecting the potential for curves to behave rather poorly and contribute to patient morbidity and discomfort. In this patient cohort, which spanned approximately 2 decades, long-term surgical correction averaged 50% using methods that have evolved more recently, owing to the advances in spinal implant technology. In our experience, more modern techniques of screw fixation and anchorage with iliac bolts to the pelvis demonstrate greater correction (>66%) and near complete correction of pelvic obliquity.
Perioperative planning
Preoperative Workup
For any surgical procedure, an appropriate history and physical examination must be obtained, as well as an understanding of the patient’s or the family’s expectations from the surgery (ie, improved sitting posture vs cosmetic correction). In addition, the assistance of a pediatrician to ensure the patient is medically optimized for surgery is very helpful. For instance, assessing the appropriate nutritional status of the patient (eg, obtaining complete metabolic panel, and prealbumin, and albumin levels) is critical for possible wound-healing complications, but may require the use of gastrostomy-tube placement preoperatively in severely nutritionally deficient patients. In addition, cardiomyopathy is progressive in the cohort of patients with DMD, although rarely is ejection fraction impaired to the level precluding surgical management of scoliosis. A prolonged impressive tachycardia is commonly seen in the DMD patient preoperatively because of the effects of dystrophin on the autonomic nervous system. Close cardiac monitoring and pulmonary interventions during the immediate postoperative period (48–72 hours) require critical care support. Preoperative cardiology consultation is helpful to assess ejection fraction and to preclude the more uncommon aberrant pathways and conduction anomalies that may be seen with involvement of Purkinje fibers. Echocardiograms and electrocardiograms are routinely obtained in these patients.
In their institutional experience with appropriate patient selection and postoperative pediatric critical care management aided by excellent anesthetic care, the authors have noted that the average length of time intubated postoperatively is less than a day and the average stay in the intensive care unit is between 2 and 3 days. All patients and families are counseled about the potential need for prolonged mechanical ventilation, the risk for postoperative pneumonias, and the remote possibility that tracheostomy may be required should weaning from mechanical ventilation be prolonged in time course.
Preoperative Radiographic Workup
AP and lateral scoliosis films are obtained along with traction and bending films, which assist in determining flexibility of the curve and the appropriate levels for instrumentation (see Fig. 1 B; Figs. 2 A, B and 3 A). A majority of neuromuscular scoliosis can be treated with posterior spinal instrumentation and fusion from T3 to the pelvis. However, in the case of an extremely rigid curve, the need for an anterior/posterior procedure is required to achieve adequate correction. Preoperative computed tomography scans may be used specifically to ascertain the bony anatomy for placement of spinal instrumentation. The use of preoperative magnetic resonance imaging is reserved for those cases whereby spinal cord abnormalities are suspected.