CHAPTER 14 Neuropathic wounds: the diabetic wound
1. Describe three types of neuropathy that may occur in the patient with diabetes.
2. Identify critical factors to be included in the history and physical examination of the patient with lower-extremity neuropathic disease.
3. Describe the correlation of protective sensation with risk for diabetic foot ulcer.
4. Distinguish from among the musculoskeletal foot deformities that lead to focal areas of high pressure in the patient with peripheral neuropathy.
5. Identify key components of a patient education program for the patient with lower extremity neuropathic disease.
6. Identify key concepts in effective and appropriate offloading.
Lower extremity neuropathic disease (LEND) develops as a result of damage to nerve structures. In the case of diabetic foot ulcers, lower extremity metabolic changes and peripheral arterial disease (PAD) exacerbate neuropathy. Diabetic foot ulcers are sometimes referred to as neurotrophic, trophic, perforating, or mal perforans ulcers (see Plate 40). The presence of possible coexisting factors, such as impaired perfusion, susceptibility to infection, neuropathy, biochemical abnormalities, repeated or continuous trauma, or a combination of these factors, in the patient with LEND who has diabetes creates a particularly challenging situation for ulcer healing (Frykberg, 2003). This chapter presents the assessment, prevention, and management of the diabetic foot ulcer.
Epidemiology
The prevalence of diabetes in the United States is 7.8% of the population or 23.6 million people (2007 estimate) and is increasing. Of these cases, 5.7 million are undiagnosed (Centers for Disease Control and Prevention [CDC], 2007). Cases of type 2 diabetes in the United States are steadily increasing. In the 12 years from 1990 to 2002, the prevalence of diagnosed diabetes doubled (CDC, 2004b). Data from 2007 indicate that 57 million adults age 20 years and older qualify as prediabetic, that is, they have fasting glucose levels that are elevated but still below the threshold for a diagnosis of diabetes (CDC, 2007). The increasing prevalence of diabetes is due to a wide variety of causes, with the obesity epidemic and an aging population heading the list. Although population-based prevalence data are lacking, statistics indicate that type 2 diabetes is on the rise among Americans 10 to 19 years old (American Academy of Pediatrics, 2009). An increase in type 2 diabetes is expected, with an estimated 7% of adolescents in the United States meeting criteria of prediabetes (CDC, 2007). These data lay the foundation for future research into the causative factors and lifetime risk of diabetes.
Using the Behavioral Risk Factor Surveillance System (BRFSS), a national survey database, the CDC (2003) estimated a 12.7% prevalence of patients with diabetes who had a history of foot ulcers. In the BRFSS, foot ulcers are defined as “any sores or irritations on the feet that took greater than 4 weeks to heal.” Reiber et al (1995) estimated a slightly higher 15% to 20% prevalence of diabetic foot ulcers during the lifetime of a patient with diabetes. Within a given year, the incidence of patients with diabetes who develop foot ulcers ranges from 1.0% to 4.1%, with a potential lifetime risk of 25% (Abbott et al, 2002; Muller et al, 2002; Singh et al, 2005).
Foot ulcers precede lower extremity amputations in 85% of cases (Larsson et al, 1998; Pecoraro et al, 1990). The leading nontraumatic cause of lower extremity amputations in the United States is attributed to diabetes. Of all the nontraumatic amputations in the United States, 50% to 75% are caused by diabetic foot ulcers (CDC, 2001). The national average annual incidence of patients with diabetes having a lower extremity amputation was approximately 4.8 per 1,000 (age-adjusted) for the year 2005 (CDC, 2008a).
After decades of steady increase, the percentage of amputations in the diabetic population compared to total amputations appears to be decreasing. Department of Veterans Affairs data show that in 1986, 59% of all amputations were because of diabetes. In 1998, 66% of all amputations were because of diabetes (Mayfield et al, 2000). In 1997 the total number of lower extremity amputations for patients with diabetes in the United States peaked at 84,000 (excluding military health care facilities), and the total figure remained above 80,000 annually until 2001 and then dropped to 71,000 in 2005 (CDC, 2004a, 2008b). Presumably, advancements in therapeutic modalities and diagnostics, implementation of multidisciplinary teams, and advanced wound care have supported this downtrend. Although overall lower extremity amputation rates are declining, they continue to rise with patient age (CDC, 2008c). The rate of lower extremity amputation for patients with diabetes is 28 times greater than for individuals without diabetes. Furthermore, amputation of the contralateral limb within 2 to 3 years is 50% to 84%, although implementation of a multidisciplinary foot care service has been shown to lower contralateral amputation rates to as low as 7% and to lower the odds ratio for lower extremity amputation in diabetics compared to nondiabetics (Van Gils et al, 1999). Driver et al (2005) reported an 82% reduction in amputations using a multidisciplinary approach, despite a 48% increase in diagnosed diabetics receiving treatment. Three years after a patient with diabetes has a lower extremity amputation, the mortality rate is 20% to 50% (Reiber et al, 1995). Five-year survival rates among patients with diabetes who undergo above-knee and below-knee amputations were as low as 28% (Aulivola et al, 2004).
Economic burden
The cost of care increases significantly when a diabetic foot ulcer proceeds to amputation. Using 2001 costs, the total event cost ranges from $23,700 for a toe amputation to $51,300 for an above-the-knee amputation (Gordois et al, 2003). An earlier, comprehensive study of amputation costs from Sweden that includes all inpatient, outpatient, and home care costs over a 3-year period estimated a cost of $43,100 for a minor amputation and $63,100 for a major amputation (Apelqvist et al, 1995). Shearer et al (2003) reported that the cost to treat an uninfected foot ulcer was $775.00 per month, increasing to $2,049.00 per month for an ulcer with cellulitis and $3,798.00 for an ulcer with osteomyelitis. Driver et al (2005) found that hospitalizations involving osteomyelitis were 2.5 times more expensive as those with no infection. Economic data support early identification and intervention of diabetic foot ulcers to prevent not only amputations but also the vast increases in costs associated with recurring infections, comorbid disease progression, and hard-to-close wounds (ADA, 2003a).
Pathogenesis
The origin and development of a diabetic foot ulcer have several components. An in-depth causal pathway study with two patient cohorts from different parts of the world identified 32 unique causal pathways for developing foot ulcers. The study found three components present in the majority (63%) of the identified pathways: peripheral neuropathy, structural foot problems, and minor trauma (Reiber et al, 1999). In another study, peripheral neuropathy was the major contributing factor leading to the development of 90% of all foot ulcers (Boulton, 1994). The risk of developing a diabetic foot ulcer is seven times more likely in diabetic patients with neuropathy than in their nonneuropathic counterparts (Rathur and Boulton, 2007). Other less prevalent causes were edema, callus, and peripheral ischemia resulting from PAD. Diabetics with PAD have been shown to have more distal disease coupled with poorer mortality and amputation outcomes than nondiabetic patients (Jude et al, 2001; Rathur and Boulton, 2007). Although infection is a common factor (59%) associated with lower extremity amputation in the patient with diabetes, it is not a common cause leading to diabetic foot ulcers (Pecoraro et al, 1990).
Neuropathy
Peripheral neuropathy is involved in 78% of diabetic foot ulcers (Reiber et al, 1999). The incidence of neuropathy in patients with diabetes appears to be linked to the duration of diabetes and, to some extent, to glycemic control. Prospective studies comparing patients with standard versus those with tighter control of blood glucose have shown that patients with better glucose control have better nerve conduction velocity as well as less retinopathy and nephropathy (Diabetes Control and Complications Research Group, 1993). Lowering hemoglobin A1c values have been shown to decrease the neuropathic and microvascular complications of diabetes (American Diabetes Association [ADA], 2007). The exact etiology of peripheral neuropathy is unknown but likely is the result of metabolic events, including accrual of glucose, sorbitol, and fructose, reduction in myoinositol (needed for nerve conduction), and nerve ischemia due to reduction in the number and diameter of vessels in the vasa nervosum (Levin, 2002). The predominant structural mechanism affected by the various metabolic components may be the microvascular component. Under normal conditions the arteriole-venule (AV) shunts in the sole of the foot are closed, and blood flows through the nutrient capillaries (capillary dermal papillae loops). With diabetic neuropathy a decrease in the sympathetic innervation of the highly innervated AV shunts results in a greater dilation in the arterioles, which leads to a shunting away of blood from the capillary dermal papillae loops. This results in lower skin temperature and a decrease in transcutaneous oxygen tension at the skin. Theoretically the metabolic components may be reversible, but structural component changes (shunting) apparently cannot be undone once changed (Tanneberg and Donofrio, 2008). However, recent advances and several randomized clinical trials in various areas of gene therapy and angiogenesis show promise in changing this long-standing convention and expanding our insight into the disease process (Driver and LeBretton, 2008). A more detailed explanation of the many etiologic pathways that lead to diabetic neuropathy is beyond the scope of this chapter.
Sensory and motor neuropathy.
Motor neuropathy affects the muscles required for normal foot movement and can result in muscle atrophy. The distal motor nerves are the most commonly affected and cause atrophy of the small intrinsic muscles of the foot. Often the wasting of the lumbrical and interosseous muscles of the foot will result in collapse of the arch (Sumpio, 2000). Cocked-up or claw toes, hammer toes (Figure 14-1), and weight redistribution from the toes to the metatarsal heads lead to increased pressures and subsequent ulceration (Levin, 2002). Generally, patients with diabetes develop both kinds of distal symmetric polyneuropathy (Sumpio, 2000).
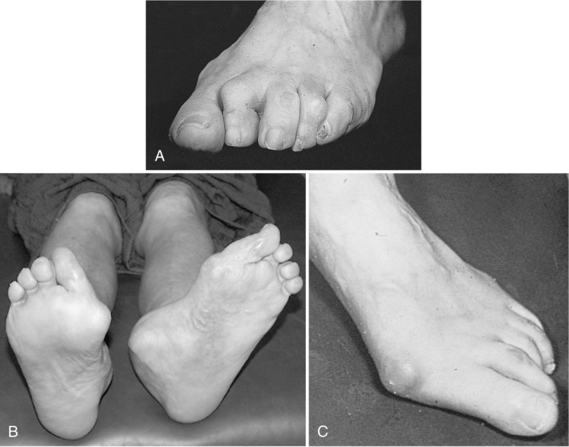
FIGURE 14-1 A, Hammer toes. B, Charcot foot. C, Hallux valgus (lateral deviation of hallux) and bunions.
(A, C From Seidel HM et al, editors: Blood vessels. In: Mosby’s guide to physical examination, ed 6, St. Louis, 2006, Mosby, Elsevier Science. Courtesy Charles W. Bradley, DPM, MPA, and Caroline Harvey, DPM, California College of Podiatric Medicine. B From Bowker JH, Pfeifer MA: Levin and O’Neal’s the diabetic foot, ed 6, St. Louis, 2001, Mosby.)
Autonomic neuropathy.
Autonomic neuropathy—a disease of the involuntary nervous system—can affect a wide range of organ systems throughout the body. Diabetic autonomic neuropathy frequently coexists with other peripheral neuropathies and other diabetic complications (Vinik et al, 2003). Autonomic neuropathy results in decreased sweating and oil production, loss of skin temperature regulation, and abnormal blood flow in the soles of the feet. The resulting xerosis can precipitate fissures, cracks, callus, and finally ulceration (Mulder et al, 2003; Tanneberg and Donofrio, 2008).
Musculoskeletal abnormalities
Foot deformities (Table 14-1) are very common in patients with diabetes and peripheral neuropathy and lead to focal areas of high pressure. These deformities are also associated with thinning of the fat pad under the metatarsal heads. Diabetic foot ulcers generally result from repetitive stress on “hot spots” that develop from bone deformities and/or callus buildup (Levin, 2002). The areas at the top of the toes, the tips of the toes, under the metatarsal heads, and the heels are vulnerable to ulceration and infection. Atrophied or dislocated fat pads beneath the metatarsal heads increase the pressure under them. This situation can lead to skin loss or callus development and increases the risk of ulceration (Sumpio, 2000). A 28% incidence of ulcers among neuropathic patients with elevated plantar pressures has been observed compared to patients with normal plantar pressures. Notably, no ulcers were observed in the normal pressure group (Boulton, 2004).
TABLE 14-1 Brief Descriptions for Selected Foot Malformations
| Malformation | Characteristics |
|---|---|
| Plantar fasciitis | Heel pain caused by inflammation of long band of connective tissue running from calcaneus to ball of foot |
| Heel spurs | Bony growths on underside, forepart of calcaneus bone; may lead to plantar fasciitis |
| Bunions (hallux valgus) | First joint of large metatarsal slants outward, with tip angling toward other toes; may lead to edema, tenderness |
| Hammer (claw) toes | Toes appear bent into claw-like position, often seen in second metatarsal when bunion slants large metatarsal toward and under it |
| Neuromas | Enlarged, benign growths of nerves, most commonly between third and fourth toes; caused by bones or other tissue rubbing against and irritating the nerves |
| Charcot arthropathy | Disruption or disintegration of some foot and ankle joints; frequently associated with diabetes, resulting in erythema, edema, deformity |
| Pes cavus | High arch or instep |
| Pes covus | Flat foot |
Associated callus can increase foot pressure by as much as 30% (Young et al, 1992). Callus presence has been associated with a 77-fold increase in ulceration in one cross-sectional study. Further follow-up data showed that plantar ulcers in neuropathic patients formed only at callus sites, suggesting an infinite risk for ulcer development (Murray et al, 1996). In the absence of neuropathy, the patient can feel the presence of a fissure, blister, or bony prominence and will take corrective action. However, with neuropathy the protective response is diminished or even nonexistent. Thus foot ulcers can get progressively worse before any action is taken. Individuals with diabetic neuropathy have been known to walk around for days in shoes containing shoehorns. Abnormalities in foot biomechanics from the previously described deformities and possible ulceration often cause a dysfunctional gait, which leads to further damage to the structure of the foot.
Ankle joint equinus.
Ankle joint equinus, defined as less than 0 degrees of ankle joint dorsiflexion, occurs in some patients with peripheral neuropathy. With ankle joint equinus the range of motion of the foot joint becomes limited, which increases pressure on the sole of the foot (Caselli et al, 2002). Of all patients with diabetes, 10.3% develop ankle joint equinus; this risk increases with duration of disease (Lavery et al, 2002). High plantar pressures from ankle equinus can increase the incidence of ulceration in patients with diabetes (Caselli et al, 2002).
Charcot foot.
Charcot foot or Charcot neuroarthropathy (or arthropathy) is a classic and increasingly common diabetic foot deformity affecting nearly 10% of diabetics with neuropathy and greater than 16% of those with a history of neuropathic ulcer (Reiber et al, 1995). Lavery et al (2003) found the incidence of Charcot arthropathy for non-Hispanic whites with diabetes to be 11.7 per 1,000 per year. A long duration of diabetes is an important factor in the development of Charcot neuroarthropathy; greater than 80% of patients with Charcot foot had diabetes for more than 10 years (Cofield et al, 1983).
Peripheral arterial disease
PAD is a major risk factor for lower extremity amputation, particularly in patients who have diabetes, because the accompanying inadequate oxygenation and perfusion of tissues significantly impair wound healing (see discussion of PAD in Chapter 11) (Mulder et al, 2003). In a comparison between patients with diabetes and patients without diabetes and PAD, patients with diabetes were five times more likely to have an amputation (Jude et al, 2001).
Relatively little is known about the biology of PAD in patients with diabetes; however, it is thought to be similar to other manifestations of atherosclerotic disease, such as coronary artery disease and carotid artery disease (ADA, 2003b). Seventy percent of deaths among type 2 diabetics can be attributed to vascular disease (National Diabetes Advisory Board, 1983). PAD typically results from gradual diameter reduction of the lower extremity arteries and from the progression of atherosclerotic changes in arterial circulation in the lower extremities. Endothelial injury and resulting endothelial dysfunction occur in the earliest stages of the disease. The endothelial surface can be injured by various means, including hyperlipidemia and diabetes (Levy, 2002). The atherosclerotic plaque that develops in the patient with diabetes and PAD is no different than the plaque that develops in the patient without diabetes (Levin, 2002). The pattern of PAD in patients with diabetes is such that medium-size arteries, mainly at the popliteal trifurcation, are affected. However, distal pedal vessels are spared (Steed et al, 2006).
Assessment
Patient history
The patient history includes general state of health, a record of diabetic complications and treatments, walking difficulties, shoe problems, pain in the extremity, medications (prescribed and over-the-counter), glycosylated hemoglobin level, and risk factors for LEND and diabetic foot ulcers. Because diabetic foot ulcers can occur as a consequence of neuropathy and lower extremity arterial disease (LEAD), specific questions regarding any LEAD risk factors should be posed (see Box 11-1).
Risk factors.
• 1.7 in persons with peripheral neuropathy
• 12.1 in persons with peripheral neuropathy and foot deformity
• 36.4 in persons with peripheral neuropathy, foot deformity, and a history of previous amputation
Foot ulcers often have a multifactorial etiology. Although the earlier studies list the most commonly associated risk factors, the clinician must recognize many other risk factors in order to comprehensively assess a patient. Box 14-1 contains a list of the most commonly recognized risk factors for ulceration (Abbott et al, 2002; Lavery et al, 1998). As with the presence of infection, vascular insufficiency has a much more important role in delaying wound healing and subsequent amputation than as a risk factor contributing to ulceration (Lavery et al, 1998).
BOX 14-1 Diabetic Foot Ulcer Risk Factors
• Absence of protective sensation due to peripheral neuropathy
• Structural deformities and callus formation
• Autonomic neuropathy causing decreased sweating and dry feet
• Past history of ulcer or amputation
• Ethnic background with high incidence of diabetes (e.g., Native American)
• Poor footwear inadequately protecting skin from high pressures
Classification of risk.
Many specialized foot treatment clinics use a foot risk classification system for patients with diabetes to allocate resources such as therapeutic shoes, education, and frequency of clinic visits (Peters and Lavery, 2001). The International Working Group on the Diabetic Foot (Apelqvist et al, 1999) recommends the “international” system as listed in Box 14-2. Risk classification systems have been shown to be very effective in predicting future diabetic foot ulcers (Lavery et al, 1998; Peters and Lavery, 2001). Additional risk classification systems are provided in Tables 14-2 and 14-3.
BOX 14-2 Foot Risk Classification by the International Working Group on the Diabetic Foot
TABLE 14-2 Foot Risk Classification System and Management Considerations
| Low-Risk Diabetes | Moderate-Risk Diabetes | High-Risk Diabetes |
|---|---|---|
| Classification | ||
| Intact sensation (neurologic) | Intact sensation (neurologic) | Absence of sensation (neurologic) |
| and/or | and/or | and/or |
| Intact pulses (vascular) | Intact pulses (vascular) | Absence of pulses (vascular) |
| Absence of foot deformities | Presence of foot deformities | Presence or absence of foot deformities |
| Management | ||
| Education emphasizing disease control, proper shoe fit/design, daily self-inspection, early reporting of foot injuries or breaks in skin | Education emphasizing disease control, proper shoe fit/design, daily self-inspection, early reporting of foot injuries or breaks in skin | Education emphasizing disease control, proper shoe fit/design, daily self-inspection, early reporting of foot injuries or breaks in skin |
| Proper fitting/design footwear with orthotics as needed | Proper fitting/design footwear with orthotics as needed; depth-inlay footwear, molded/modified orthosis may be required | May require modified or custom footwear |
| Annual follow-up for foot screening | Routine follow-up every 6 months for foot examination | Routine follow-up every 1–12 weeks for foot ulcer evaluation and callus/nail care |
| Follow as needed for skin/callus/nail care or orthosis | Referral to foot and ankle care specialist if deformity is causing pressure point and conservative measures fail | Referral to foot and ankle care specialist |
Data from Driver VR, Madsen J, Goodman RA: Reducing amputation rates in patients with diabetes at a military medical center: the limb preservation service model, Diabetes Care 28(2):248-253, 2005.
TABLE 14-3 Lower Extremity Amputation Prevention Program (LEAP) and Management Categories for the Foot
| Risk Categories | Definition | Management Categories |
|---|---|---|
| 0 | No loss of protective sensation of the feet | Education emphasizing disease control, proper shoe fit/design Follow-up yearly for foot screen Follow as needed for skin, callus, nail care, or orthosis |
| 1 | Loss of protective sensation of the feet | Education emphasizing disease control, fit/design, daily inspection, skin/nail care, early reporting of foot injuries Proper fitting/design footwear with soft inserts/soles Routine follow-up every 3–6 months for foot/shoe examination and nail care |
| 2 | Loss of protective sensation of the feet with either high-pressure deformity or poor circulation | Education emphasizing disease control, proper shoe fit/design, daily inspection, skin/nail care, early reporting of foot injuries Depth-inlay footwear, molded/modified orthoses Modify shoes as needed; footwear with soft inserts/soles Routine follow-up every 1–3 months for foot/activity/footwear evaluation and callus/nail care |
| 3 | History of plantar ulcer or neuropathic fracture | Education emphasizing disease control, proper fitting footwear, daily inspection, skin/nail/callus care, early reporting of foot injuries Depth-inlay footwear, molded/modified orthoses; modified/custom footwear, ankle footwear orthoses as needed Routine follow-up every 1–12 weeks for foot/activity/footwear evaluation and callus/nail care |
Note: “Loss of protective sensation” is assessed with a Semmes-Weinstein monofilament examination using a 5.07 monofilament at nine locations on each foot. Foot clinic visit frequency may vary based on individual patient needs.
From WOCN Society: Guideline for management of wounds in patients with lower-extremity neuropathic disease, WOCN Clinical Practice Guideline Series #3, Glenview, Ill, 2004.
Lower extremity and foot physical examination
Chapter 10 describes how to conduct a comprehensive lower extremity assessment and should be carefully reviewed. The following section discusses the aspects of the lower extremity examination that are unique to the patient with LEND.
Protective sensation.
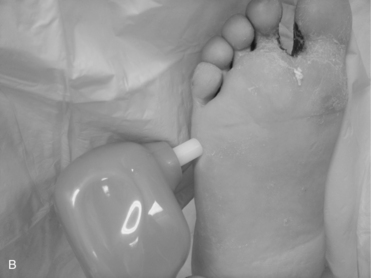
Screening for neuropathy can be done rapidly and reliably using a Semmes-Weinstein 5.07 (10-g) monofilament test or a vibration tuning fork test with the on–off method (ADA, 2007; Perkins et al, 2001). Biothesiometry expands on the traditional tuning fork, allowing for quantification of the vibration threshold (Figure 14-2, A). An electric oscillator is applied to the traditional assessment landmarks (Figure 14-2, B) and “dialed in” until the patient is able to perceive the vibration (Figure 14-2, C). The value obtained can be used to follow the progression of neuropathy and qualify future assessment findings. As shown in Figure 14-3, the monofilament line used for the Semmes-Weinstein test is normally mounted on a rigid paper holder. The line has been standardized to deliver a 10-g force when pushed against an area of the foot. Regardless of which method is used, the patient should be placed in a room that is quiet and relaxed. Boxes 14-3 and 14-4 provide procedures for conducting these examinations.
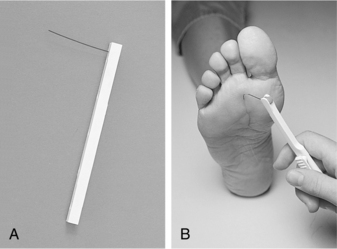
FIGURE 14-3 A, Monofilament. B, Press the monofilament against the skin hard enough so that it bends.
(From Seidel HM et al, editors: Blood vessels. In: Mosby’s guide to physical examination, ed 5, St. Louis, 2003, Mosby.)
BOX 14-3 Procedure for Semmes-Weinstein 5.07 (10-g) Monofilament Examination (SWME)
1. Explain procedure to patient.
2. Position patient in sitting position, resting patient’s lower leg on examiner’s lap.
3. Demonstrate monofilament on patient’s hand so that he or she knows what to expect.
4. Explain to patient to that he or she should respond with a “yes” when he or she feels the filament touching the skin.
5. Have patient close his or her eyes. Sites to be tested should be shown on examination form.
6. Apply monofilament perpendicular to skin’s surface. Apply sufficient force to cause the filament to buckle or bend. Use a smooth, not jabbing, motion.
7. Total duration of the approach, skin contact, and departure of filament from each site should be approximately 1–2 seconds.
8. Apply filament along margin of callus, ulcer, scar, and/or necrotic tissue; do not apply filament over these lesions.
9. Record and, if appropriate, map the results on examination form.
BOX 14-4 Procedure for Biothesiometry Examination (Amplitude of Vibration)
1. Explain procedure to patient.
2. Position patient in sitting position, resting patient’s lower leg on examiner’s lap.
3. Demonstrate oscillator on patient’s hand so that he or she knows what to expect.
4. Explain to patient that he or she should respond with a “yes “ when he or she feels the oscillator vibrating.
5. Have patient close his or her eyes. Sites to be tested should be shown on examination form.
6. Apply oscillator perpendicular to (flat against) skin’s surface. Apply just enough force for the oscillator head to make total contact with skin. Use a smooth, not jabbing, motion.
7. The amplitude of the vibration should be slowly increased until the patient perceives the vibration and the value on the meter noted.
8. Apply oscillator along margin of callus, ulcer, scar, and/or necrotic tissue; do not apply oscillator over these lesions.
9. Record and, if appropriate, map the results on examination form.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree