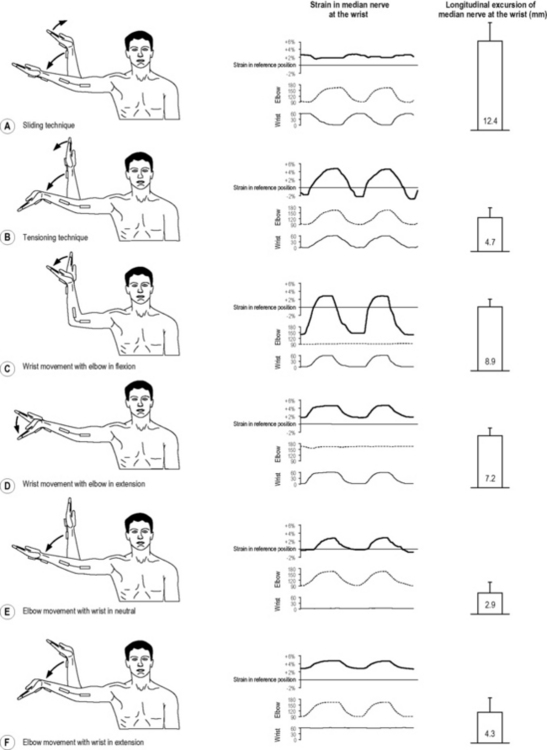
7.19 Neurodynamics The nervous system is a remarkable organ system. In many ways, it is well protected and strong. On average, 50% of a peripheral nerve consists of connective tissue (ranging from 22% to as high as 80%) (Sunderland & Bradley 1949). In addition, there are many other anatomical design features that enable the nervous system to handle the significant mechanical demands that are placed upon it during activities. Complete nerve lesions, such as nerve root avulsions, are rare and require a high-impact or high-velocity trauma. Considering the relatively low prevalence of acute traumatic nerve injury, it is somewhat surprising that the classification system for nerve disorders most commonly used to date was developed more than 60 years ago during World War II. Seddon’s classification describes three basic types of nerve injuries according to severity: neuropraxia, axonotmesis and neurotmesis (Seddon 1943). It is important to emphasize that this grading scheme and Sunderland’s modification of the scheme (Sunderland 1951) are both based on gross pathology and loss of nerve function (impulse conduction). Negative symptomatology, such as loss of sensation (numbness) and loss of muscle strength (weakness) can be easily explained by the varying degrees of demyelination and axonal loss as described for the different categories. However, important positive symptomatology, such as neuropathic pain, is not properly accounted for in these classifications. Sunderland acknowledged that many peripheral neuropathies may exist where altered nerve conduction may not be severe enough to be classified as a neuropraxia (Sunderland 1978). Due to a lack of understanding at the time, he could only refer to these disorders as ‘irritative lesions’ or ‘perverted nerves.’ Thanks to advances in neuroscience over the last decades, a much better knowledge of the pathophysiology of these nerve injuries is now available. Before discussing movement as a treatment modality for neuropathic pain states, we need to briefly review the structure, function and pathophysiology of the peripheral nervous system. The connective tissue sheaths are innervated by the nervi nervorum (Sauer et al. 1999). The nerve trunk is also sympathetically innervated via the perivascular plexuses of the blood vessels which enter the nerve (Bove & Light 1997). The small nervi nervorum are predominantly unmyelinated, and at least some function as nociceptors. As a result, the connective tissues may contribute to a pain experience, theoretically irrespective of pathological changes in conductive nerve fibers. A very well-developed system of extraneural and intraneural blood vessels supplies the nerve. The blood vessels pierce the different connective tissue sheaths to provide oxygen and essential nutrients to the cells. The endothelium cells which line the interior surface of the endoneurial capillary bed, together with the perineurium, form the blood–nerve barrier (Yayama et al. 2010). Similar to the blood–brain barrier, it is designed to keep unwanted substances out of the perineurial space. Compression and intraneural ischemia (Yayama et al. 2010) and intraneural activated immune cells (Spies et al. 1995) may cause focal breakdown of the blood–nerve barrier. Sunderland (1976) described three pathological stages following persistent pressure in or around the nerve: hypoxia, edema and fibrosis. Pressure may lead to venous stasis. Ischemia and hypoxia may cause pain and other symptoms, such as paresthesia. If hypoxia continues, the blood–nerve barrier will break down, resulting in accumulation of proteins and edema. Because no lymphatic vessels cross the blood–nerve barrier, it may take a long time for edema within the perineurium and endoneurium to be reabsorbed (Rempel et al. 1999). Lymphocytes, fibroblasts, and macrophages will intrude as a reaction to previously shielded antigens contained within the perineurial space. This will initiate inflammation and eventually fibrosis or scar formation in the subperineurial space. Thickening of the epineurium and perineurial connective tissue sheath is also observed (Mackinnon 2002). As a comprehensive overview of nerve pathophysiology is beyond the scope of this chapter, the reader is referred to selected reviews for further information (Rempel et al. 1999; Mackinnon 2002). The double crush theory states that axons which are compressed in one region become susceptible to damage at another site (Upton & McComas 1973). The basis for this hypothesis was the observation of a high prevalence of cervical radiculopathy in patients with CTS (5–18%) (Morgan & Wilbourn 1998; Moghtaderi & Izadi 2008), compared to less than 1% in the general population (Radhakrishnan et al. 1994). Although first formulated almost 40 years ago, little is known about possible underlying mechanisms to support the theory, and the existence of the phenomenon is often questioned. In a recent Delphi study (Schmid & Coppieters 2010), we asked a panel of international experts in peripheral nerve pathology to indicate their level of agreement with the statement that a nerve disorder is a predisposition for the development of a second nerve disorder. Two-thirds of the experts either agreed or strongly agreed with the statement, whereas one-third disagreed. When asked to list mechanisms to explain dual or multiple nerve injuries, the experts listed 22 possible processes. Previously suggested mechanisms such as impaired axonal transport or altered nerve biomechanics were included. In addition, many new mechanisms not previously linked to dual nerve disorders were suggested, such as ion channel alterations and immune-inflammation of the peripheral nerve, dorsal root ganglion, spinal cord, and higher pain centers. Many clinicians have embraced the double crush theory as it provides a rationale for considering the health of the entire nervous system and its surrounding structures when examining and treating patients with neuropathic pain. It has been argued that failure to diagnose and treat these multiple levels of injury will result in failure to relieve patients’ symptoms (Mackinnon 2002). However, due to a lack of research, clinical guidelines refrain from making treatment recommendations for patients with dual nerve disorders, such as cervical radiculopathy and CTS (American Academy of Orthopaedic Surgeons, 2008), and much basic research is needed before possible mechanisms for dual nerve disorders can be substantiated. There is relatively little research that has investigated the effects of movement on neuropathic pain, especially studies involving humans. A recent animal study revealed that an extended exercise program reduced signs of neuropathic pain in rodents who had sustained partial sciatic nerve injury (Kuphal et al. 2007). Following exercise, there was a reduction in cold allodynia and thermal hyperalgesia. In this section, the focus will be on one type of therapeutic movement, namely neurodynamic exercises or nerve gliding exercises. Historically, the first mobilization techniques for the nervous system resembled neurodynamic tests, or ‘tension tests’ as they were initially called. Several biomechanical studies have revealed that the nervous system slides considerably relative to its surrounding structures and that strain in the nervous system increases substantially during neurodynamic tests or individual components of the test (Byl et al. 2002; Wright et al. 2005; Coppieters et al. 2006; Gilbert et al. 2007; Coppieters & Butler 2008). Because of the increase in strain, these initial mobilization techniques are now often referred to as ‘tensioning techniques’ (Butler 2000). Examples of tensioning techniques for the median nerve are elbow extension combined with wrist extension (Fig. 7.19.1B), elbow extension with cervical contralateral side bending (Fig. 7.19.2B), or the combination of wrist and finger extension (Fig. 7.19.3). Obviously, exercises can include various combinations and can be much more functional than the techniques mentioned here, which have been subjected to biomechanical analysis (see below).
Movement for neuropathic pain states
Introduction
Structure, function and pathophysiology of the peripheral nervous system
The double crush theory
Movement for neuropathic pain states
Neurodynamic exercises: ‘sliders’ slide and ‘tensioners’ tension
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neurodynamics: Movement for neuropathic pain states