11. Myofascial pain syndrome—myofascial trigger points
Myofascial pain syndrome (MPS) is one of the most common causes of pain stemming from soft tissue dysfunction (Alvarez & Rockwell 2002), and can be described as pain from muscle tissue with surrounding fascia. Chronic myofascial pain is often a result of both physical and psychosocial influences that may complicate convalescence (Wheeler 2004). It is estimated that around 10–15% of the US population have some form of myofascial pain (Alvarez and Rockwell, 2002 and Wheeler, 2004). The prevalent occurrence and effects also make it a valid concern for athletes. Myofascial trigger points (MTrPs) are considered to be the main cause of MPS (Bonci, 1993 and Dommerholt et al., 2006), and it is suggested that MTrPs can affect athletic performance in negative ways, even when not actively producing actual pain sensations (Bonci 1993). Several probable mechanisms can cause the formation of MTrPs, including direct trauma, low-level muscle contractions, uneven intramuscular pressure distribution, unaccustomed eccentric contractions, eccentric contractions in unconditioned muscles, and maximal or submaximal concentric muscle contractions (Dommerholt et al. 2006).
A myofascial trigger point can be described as a hypersensitive focal nodule in a taut band of skeletal muscle fibers. MTrPs generally present motor sensory (Alvarez and Rockwell, 2002, McPartland, 2004 and Dommerholt et al., 2006) and referred autonomic symptoms (Simons, 1999 and Dommerholt et al., 2006). Sensory disturbances may include local tenderness, allodynia, hyperalgesia (see Box 11.1), and referred pain to a distant area and/or certain muscles unique for each MTrP (Dommerholt et al. 2006). Motor symptoms can manifest as disturbed motor function, muscle weakness from inhibition, limited range of motion, and muscle stiffness.
Box 11.1
• Acetylcholine (ACh)—neurotransmitter activating skeletal muscle contraction and the autonomic nervous system.
• Acetylcholinesterase—enzyme breaking down ACh.
• Adenosine triphosphate (ATP)—a “stored energy form” utilized by the cells.
• Allodynia—pain from a stimulus that does not normally provoke pain.
• Ca2+—calcium ions that stimulate contraction of skeletal muscle cells.
• Dorsal horn—site of spinal cord receiving and processing incoming sensory information.
• Hyperalgesia—elevated reaction to a stimulus that is normally painful.
• Hypothyroidism—disease caused by insufficient production of certain hormones by the thyroid gland.
• Motor endplate—flat end part of a motor neuron transmitting nerve impulses to a muscle cell.
• Nociceptive—responding to a physiological pain stimulus.
• Reversed T3 (rT3) —a mostly inactive thyroid hormone that can still occupy receptors dedicated for the active T3 hormone.
• Sarcolemma—cell membrane of a muscle cell.
• Sarcomere—smallest contractile unit in a muscle cell, consisting of thin actin and thick myosin protein filaments.
• Sarcoplasmic reticulum (SR)—“tubular network” in the muscle cells. The end sacs of the SR store calcium ions vital for muscle contraction.
• Vasoconstriction—constriction of certain blood vessels.
MTrP and taut band
The taut band “housing” the MTrP is found in an otherwise more relaxed muscle (Fig. 11.1). The band forms by tension generated from contracted and stretched sarcomeres relating to a more centrally located knot (Simons & Dommerholt 2006a). It is important to note that MTrPs will always be found in a taut band, but a taut band does not always contain a MTrP.
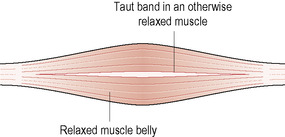 |
| Figure 11.1 |
MTrPs are generally divided into active and latent MTrPs. Both active and latent MTrPs are normally painful upon compression. The pain referral zone is commonly projected to a distant area, sometimes without necessary spontaneous pain sensations at the actual site of the trigger point itself.
The exact etiology of MTrPs has yet to be fully established (Simons 2008), but different theories have been presented over the years.
MTrPs and trauma
It is suggested that some form of trauma initially damages the end sacs of the sarcoplasmic reticulum (SR) and the sarcolemma. The trauma is either acute or in the form of repetitive microtrauma caused by overuse or overload of local muscles, something heavily prevalent in the athletic community. This produces a substantial release, with increased intracellular concentration, of calcium ions (Ca2+), triggering contractions of local sarcomeres in the affected muscle cells (Fig. 11.2).
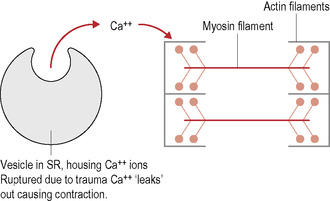 |
| Figure 11.2 |
More recent research has formed the generally adopted theory named “the integrated trigger point hypothesis” (Simons et al. 1999; Fig. 11.3). It suggests that an abnormal release of acetylcholine (ACh) from the motor endplates, even during rest, produces a sustained depolarization of the affected muscle cells. ACh triggers the calcium channels in the SR to start releasing Ca2+. An impaired release of acetylcholinesterase is also found to play a role (Simons 2008).
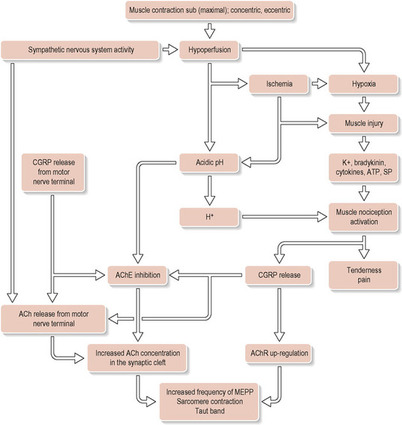 |
| Figure 11.3 Reproduced with permission from Gerwin et al. 2004 |
The malfunctioning motor endplates form a “central MTrP” located at the innervation site of the muscle. MTrPs seems to begin in a region of multiple dysfunctional endplates with an abnormal release of ACh. The dysfunctional endplates are connected to a section of muscle fibers forming a contractile knot and taut band in an otherwise more relaxed muscle. The sustained contraction increases local metabolic demands and constricts local capillaries, which reduces available oxygen and nutrient levels in the area, limiting ATP production. An accumulation of metabolites triggers local vasoconstriction that further decreases blood circulation. This finally leads to a local energy crisis due to a lack of ATP. The calcium pump returning calcium into the end sacs of the SR is highly dependent on ATP. The sudden lack disrupts its function, potentially sustaining the localized contractile reaction (Simons et al. 1999). The energy crisis triggers release of substances, i.e. bradykinin, cytokines, serotonin, histamine, E-type prostaglandins, substance P, etc., causing increased pain sensation. It is thought likely that if the endplate dysfunction is allowed to continue for any length of time, chronic fibrotic changes may occur in the tissue (Hou et al. 2002).
Contributing additional factors to MTrP development are deficiencies in vitamins B1, B6, B12, C, D, and folic acid, magnesium, zinc, and iron (Dommerholt et al. 2006; Simons & Dommerholt 2006a) as well as hormonal dysfunctions like hypothyroidism, increased levels of reversed T3, and human growth hormone deficiency (Simons & Dommerholt 2006a). Depressive emotional states have also been shown to predispose people to MTrP formation (Simons et al. 1999).
Pain and myofascial trigger points
Because MTrP pain does not project along regular dermatomes, the exact explanation model for referred MTrP pain is still somewhat uncertain, although particular neural explanation models are favored. Some correlations between MTrPs and acupoints have also been mapped, and more recent investigations suggest the possibility that MTrPs and Ah Shi points could be the same phenomenon (Birch 2003; Simons & Dommerholt 2006b). It has also been shown that drawings by patients describing their pain pattern coincided to a high degree with the pathways of acupuncture channels (Baldry 1993).
Reproduction of the local or referred pain is possible through mechanical stimulation of a MTrP, either by focal compression or stretching. The pain may appear immediately or after a 10–15s delay. Although distinctive referred pain patterns for each MTrP have been established, substantial variations exist (Dommerholt et al. 2006).
Convergence–projection
This theory is one of the more widely adopted models of explanation (Baldry, 1993 and Simons, 1999). It is considered that cutaneous, visceral, or skeletal muscle afferent nerve fibers converge in the same spinal neuron. The referred pain patterns from MTrPs are not automatically restricted to regular peripheral dermatome nerve distributions or single segmental pathways. MTrPs cause constant irritating stimulation, increasing both the size and number of receptive areas to which a single dorsal horn nociceptive neuron may respond, and this affects the referred pain sensation (Dommerholt et al. 2006). It is believed that the sensory cortex of the brain could possibly misinterpret a strong visceral or skeletal muscular input as stemming from the corresponding skin site.
Sympathetic hyperactivity
The referred pain area of MTrPs can have lowered skin temperature, induced by sympathetic hyperactivity causing constriction of superficial blood vessels (Baldry 1993). This may lead to the release of chemical sensory afferent sensitizing substances, which could generate pain in a local pain zone.
Classification of myofascial trigger points
Active MTrPs
Active trigger points refer pain, other paraesthesias (Dommerholt et al. 2006), and/or cause referred autonomic phenomena when the muscle is contracted, stretched, or during rest. Active MTrPs are always tender and prevent full lengthening of the muscle. One example of pain caused by active trigger points is tension-related headaches. During a treatment it is common that an active trigger point is reduced to a latent trigger point. The pain is relieved, but the taut band with the MTrP still remains, and is considered completely cleared only when the taut band has dissolved.
Latent MTrP
Latent trigger points do not cause spontaneous referred pain or autonomic phenomena unless stimulated; however, they do still render the muscle shorter and weaker than normal since the taut band and dysfunction are present. Latent trigger points often shift into active trigger points when the muscle is overloaded.
Central MTrP
A central trigger point is closely associated with dysfunctional motor end plates and is located at or near the center of a muscle belly where the nerve innervates the muscle (Simons et al. 1999). Central MTrPs are generally treated first.
Attachment MTrP
Attachment trigger points arise near the musculotendinous junction and/or at the bony origin or insertion due to the strain at the attachments from the formed taut band (Simons et al. 1999). If the strain is more intense, two attachment trigger points may form at the site, with one at the musculotendinous junction and one at the tendon–bone junction.
Key trigger points
Key trigger points activate one or more satellite trigger points (Simons et al. 1999). Deactivating a key MTrP may automatically deactivate its satellite MTrPs.
Associated trigger points
Associated trigger points occur concurrently with a trigger point in another muscle (Simons et al. 1999). One may have induced the other, or they may both stem from the same mechanical or neurological origin.
Satellite trigger points
A satellite trigger point is a central trigger point formed in a muscle located within the referred pain zone of a key trigger point (Simons et al. 1999). Satellite trigger points can also appear in overloaded synergistic muscles compensating the trigger point muscle, or in an antagonistic muscle countering increased tension from the muscle with a key trigger point.
How to localize MTrPs
MTrPs are generally located by evaluation of the skin condition, presence of taut band, local or referred pain, reflex/reactions, and/or referred autonomic phenomena.
Increased skin moisture over the MTrP
By slowly and lightly dragging one finger across the skin over the troubled area, greater resistance is noted over a MTrP. This is caused by the increased skin moisture the MTrP induces (Chaitow & Delany 2000; Fig. 11.4).
 |
| Figure 11.4 |
Reduced skin motility over the MTrP
The skin and fascia over an area of dysfunction tend to be less mobile compared with a healthy site (Chaitow & Delany 2000). By gently pushing the skin in different directions, resistance can be noted at the dysfunctional site (Fig. 11.5).
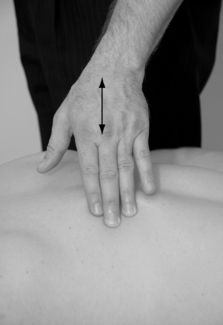 |
| Figure 11.5 |
Palpation
To localize the MTrP, the therapist also palpates the muscle in search of the taut band of muscle fibers. Pressure of the taut band in the affected muscle is often a sure diagnosis.
Locating the taut band
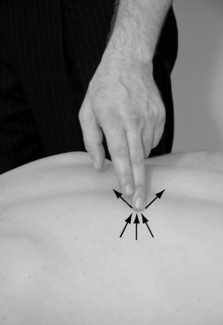
The taut band is located by moving the fingers transversally to the muscle fibers without sliding on the skin, or by grasping a muscle belly between the fingertips, gently rolling the tissue between the fingers (Fig. 11.6). Once located, the band is further carefully pressed or squeezed along its length in search of the hypertender trigger point, first centrally in the muscle belly, followed by pressure at the attachments. There are three common ways to palpate:
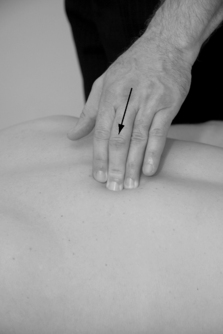 |
| Figure 11.6 |
• flat palpation
• pincer palpation
• deep/probing palpation.
Flat palpation
Flat palpation is executed with the fingertips, over muscles lacking a graspable edge, for example infraspinatus and erector spinae muscles (Fig. 11.7). Moving the skin, without sliding, perpendicular to the fiber direction helps locate the taut band.
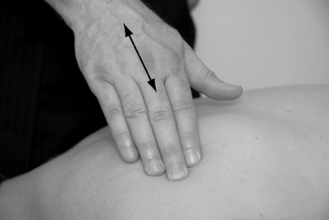 Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





