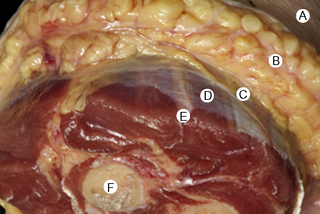
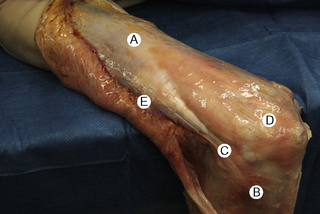
7.4 Myofascial induction approaches Myofascial Induction Therapy (MIT) is a hands-on, full-body approach, focusing on restoration of altered fascial tissue function. During the application, the clinician stretches or compresses the specific region in order to transmit a low-intensity mechanical input. These actions modify fascial restrictions in order to adjust the tension distribution in the fascial network. It is hypothesized that this procedure can restore the ability to move more efficiently and to achieve better functionality with lower energy expenditure (Useros et al. 2008). Fascial restriction is described as any impediment to optimal gliding, at both macroscopic and microscopic fascial organizational levels, between endofascial fibers and interfascial planes. Such restriction can cause anomalous tension and movement disorders (Fourie 2008). One possible reason for a restriction of the fascial tissues may be excessive stimulation of collagen production inducing fibrosis, resulting in loss of its smoothness and/or isotropy, and creation of entrapment areas. This suggests that such entrapment areas may alter physiologic body movements in relation to amplitude, velocity, resistance, and coordination (Fourie 2008). In the presence of long-term restrictions, fascial tissue becomes overloaded and suffers dysfunctional consequences. These changes first affect the loose connective tissues, followed by reorganization of regular or irregular dense connective tissue, such as tendons, ligaments, or capsules, creating excessive density and reorientation of fibers. Fascial restrictions of short durations affect the tissues locally, whereas restrictions of long duration induce a more global dysfunction pattern (Langevin 2006). Fascial tissue is related to the interchange of body fluids and to mechanoreceptor coordination. A decrease of fascial mobility can alter the blood circulation and cause ischemia, deteriorating muscle fiber quality. And since many mechanoreceptors are embedded within fascia, altered proprioceptive afferents can change the ability of optimal muscles tonic contraction (Vaticon 2009). As a result, alterations in stabilizing functions, as well as in the coordination of joint movements, may occur, with consequent difficulty of joint compression at its optimal point of action, possibly leading to joint overload, inflammation and/or pain in myofascial structures (Lee 2001). Some theories suggest that the three-dimensional fascial network can be involved in pain transmission, with peripheral pain possibly having an origin in the connective tissue (Liptan 2009; Han 2009). Taguchi et al. (2009) suggest that the thoracolumbar fascia is an important source of nociceptive input in chronic LBP patients. Various concepts exist related to the manual treatment of fascial system restrictions, with different names used to describe similar treatment approaches (Chaitow & Delany 2002; Myers 2003). The conceptual bases of most of these are similar. Further clinical research is needed in order to unify and validate these clinical procedures (Remvig 2007). Anatomical analysis in unembalmed specimens confirms continuity of the fascial system through the entire body (Fig. 7.4.1). Benjamin (2009), Stecco et al. (2008), Pilat (2009), Pilat (2010), Myers (2003), van der Wal (2009), Mass and Sandercock (2010), all demonstrate movement continuity on macroscopic levels, focused not only on the fascia-to-bone connections, but also on the direct fascia-to-fascia transmission, in both articular and intermuscular connections (Fig. 7.4.2). Ingber, (Wang et al. 2009) and Langevin (2010), among others, have demonstrated dynamic continuity at the microscopic, intracellular and intercellular levels. In 1997, Ingber proposed intercommunication systems based on tensegrity principles (Ingber 1997; Pilat & Testa 2009). This suggests a system of shared tensions in the distribution of mechanical forces, at multiple body levels, possibly explaining the global reaction of the fascial system in response to mechanical stimuli (Khalsa et al. 2000). Different studies (for example, Wang et al. 2009) have shown that cell dynamic and active responses of the cytoskeleton, responding to mechanical forces from the extracellular matrix, induce tissue remodeling at both cellular and subcellular levels. Taking into account that the structure of the body follows the principles of hierarchical assembly, the above process is not limited to cells, but also involves tissues and organs (Huang & Ingber 2000). Ingber, (Wang et al. 2009) showed continuity of mechanical stimuli from the cytoskeleton to intranuclear level, and Langevin (2010) demonstrated mechanical impulse continuity from the skin to the nucleus membrane in mouse fibroblasts. It is hypothesized that mechanical stimuli can create at least three types of reactions: • Piezoelectricity: This is a phenomenon exhibited by certain crystals that, when subject to mechanical tensions, acquire a polarization in their atomic structure, generating a difference of electrical potential and loads at their surface (Pilat 2003). The basic properties of the organism (i.e., elasticity, flexibility, elongation, resistance) depend to a great extent upon the ability to maintain a continuous information flow. Oschman (2003) affirms that information is transmitted electrically through the connective tissue matrix. Since collagen may be interpreted as a semiconductor (Cope 1975), it may be capable of forming an integrated electronic network enabling the interconnection of all fascial system components (O’Connell 2003). Further investigation is needed to evaluate how MIT may influence this property of the body network. • Dynamics of the Myofibroblasts Response: Muscles comprise contractile tissues that enable the body to move. Fascia should be considered as an intramuscular connective tissue that forms a functional unit with muscle fibers. The fascial system is highly innervated by mechanoreceptors (Stecco et al. 2008). Consequently, mechanical input (manual pressure or traction) received by mechanoreceptors may create a broad range of responses in the fascial system that facilitate movement. Studies focusing on skin healing processes and pathologies such as Dupuytren’s contracture, plantar fasciitis, frozen shoulder, that relate to actin microfilament contraction, strongly support this reasoning (Gabbiani 2007). Chaudhry et al. (2008), using a 3-D mathematical model for deformation of human fascia, suggest that mechanical forces applied during manual techniques can create mechanical changes in loose connective tissue (i.e., superficial nasal fascia). Schleip (2005) suggests that changes in resting tone of skeletal muscle fibers can transmit their tension force to the fascial tissue. • Viscoelasticity: The viscoelastic properties of fascia have been observed in numerous studies: thoracolumbar fascia (Yahia et al. 1993), fascia lata (Wright & Rennels 1964), subcutaneous fascia of rats (Latridies et al. 2003), the fascia lata, plantar fascia and nasal fascia (Chaudhry et al. 2008). Concepts for practical treatment applications have been defined by Rolf (1994), Threlkeld (1992), Barnes (1997), Cantu and Gordin (2001), Pilat (2003), and Pilat (2009). The applications of MIT suggested below are based on the clinical experience of the author (Pilat 2003), and are based on theoretical frameworks discussed above. MIT may be combined with other manual therapy strategies, or an exclusive treatment procedure. • The evaluation of fascial dysfunction should be included into clinical reasoning processes. An exhaustive case intake is required, with annotations concerning the duration of symptoms and a visual analog scale (VAS) evaluation. • We suggest automatic exploration of general posture disorders and local dysfunction testing. • Biomechanically, the myofascial system responds to compression and traction forces (Chaudhry et al. 2008). These two mechanical strategies can be used when applying MIT. • The direction of the releasing movement is towards restriction barriers. Arbitrary directions of tissue engagement should be avoided. Restrictions may occur in various directions and planes. They may also occur in different directions in the same plane, or in the same direction in various planes, or in different planes in various directions. See also Chapter 7.5 for discussion of indirect myofascial release methods. • There is no need for active muscle contraction by the patient, who may be asked to maintain a state of active passivity.
Introduction
Neurophysiologic mechanisms for releasing the restrictions of the fascial system
Method description
General observations for clinical applications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Myofascial induction approaches