Fig. 12.1
Main purposes and resources of the MDCTN
As of April 2015, 33 clinical institutes distributed over all four main islands of Japan have joined the MDCTN (Fig. 12.2); these comprise 17 hospitals of the National Hospital Organization, one national center (NCNP), 12 university hospitals and institutes, one prefectural hospital, one hospital of a local welfare organization, and one private hospital.

Fig. 12.2
Locations of the member institutes of the MDCTN. As of April 2015, 33 institutes located on all four main islands of Japan had joined the MDCTN
According to the data from the annual site registry as of December 2014, approximately 6000 patients with neuromuscular diseases visit MDCTN member hospitals annually. In decreasing order, their neuromuscular diseases are Duchenne muscular dystrophy 29 %, myotonic dystrophy type 1 21 %, limb-girdle muscular dystrophy 11 %, Becker muscular dystrophy 10 %, facioscapulohumeral muscular dystrophy 6 %, Fukuyama congenital muscular dystrophy 6 %, spinal muscular atrophy 6 %, congenital myopathy 4 %, mitochondrial disorder 4 %, congenital muscular dystrophy excluding Fukuyama type 3 %, and GNE myopathy 2 % (Fig. 12.3).
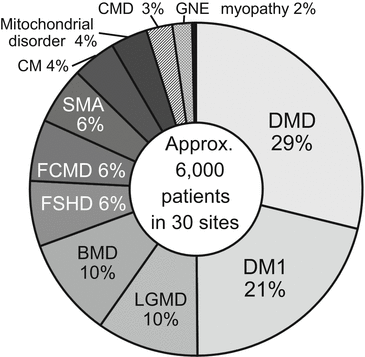

Fig. 12.3
Composition of overall patient cohort under the auspices of the MDCTN. As of December 2014, approximately 6000 patients with neuromuscular diseases were visiting the 30 MDCTN member hospitals annually. BMD Becker muscular dystrophy, CM congenital myopathy, CMD congenital muscular dystrophy excluding Fukuyama type, DMD Duchenne muscular dystrophy, DM1 Myotonic dystrophy type 1, FCMD Fukuyama congenital muscular dystrophy, FSHD facioscapulohumeral muscular dystrophy, LGMD limb-girdle muscular dystrophy, SMA spinal muscular atrophy
Pulmonary function testing, continuous pulse oximetry, standard 12-lead electrocardiography, and Holter electrocardiography are available for functional evaluation in all member institutes. Additionally, blood gas analysis, transthoracic echocardiography, electromyography, muscle magnetic resonance imaging, muscle biopsy, 6-min-walk testing, 10-m-timed walk testing, and timed up-and-go testing are available in more than 90 % of member institutes. Institutional review boards have been established in all member institutes.
12.5 The Activities of MDCTN in Japan
12.5.1 Propagation of Information and Education
The MDCTN holds annual workshops to share opinions and information regarding updates in research and current and promising therapies, these workshops being open to network members, pharmaceutical companies, patient advocacy groups, members of regulatory agencies, and anyone else with an interest in MDCTN activities. The MDCTN also has regular meetings with representatives of member institutes for communication purposes. As a component of its working group activities, the MDCTN also holds seminars about neuromuscular diseases and standardized evaluation for physical therapists and clinical research coordinators. These seminars and workshops are generally well received because they provide a great opportunity for communication between physical therapists and clinical research coordinators from different institutes.
12.5.2 Feasibility Surveys for Clinical Trials
The MDCTN has built the capacity to eliminate the need for researchers and pharmaceutical companies to spend their valuable time looking for cooperative researchers and recruiting patients. Pharmaceutical companies that have asked the secretariat office of the MDCTN to investigate the feasibility of clinical trials on muscular dystrophy have had answers within a few weeks. This is possible because the MDCTN has compiled appropriate forms that are sent directly to possible principal investigators who specialize in muscular diseases and are interested in participating in clinical trials.
12.5.3 Support for Recruitment of Patients for Clinical Trials
Two investigator-initiated clinical trials on muscular dystrophy asked the MDCTN for support in recruitment of patients with certain genetic mutations through the patient registry, Remudy [13]. Patient recruitment for one of these studies was completed within only 2 months. However, at the time of writing, the MDCTN was still working on recruitment for the other study because some possible participants were excluded by more detailed exclusion criteria or because they lived too far from the participating site. Otherwise, participants are assigned quickly from the start.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







