Miscellaneous Fungal Infections
Michael B. Smith
Michael R. McGinnis
Numerous reports have implicated soil-borne fungi as causative agents of fungal diseases in a variety of clinical settings. Because most soil- and airborne fungi are rapid growers on culture media and may overgrow cultures from lesions resulting from nonfungal causes, the vast majority of reports published in the literature relating these fungi to disease are questionable. Rarely, however, a fungus not normally considered a pathogen may cause disease in particular circumstances. What should be remembered is that strain variation in fungi is great, as are types and degrees of compromised states, and some genetic changes may enhance pathogenic potential of otherwise harmless organisms in some patient populations. Distinguishing among infection, colonization, and contamination becomes critical in deciding on patient management.
Infection occurs when the fungus invades and grows within viable tissue and sterile body fluids. The body’s response takes the form of signs and symptoms, which is disease. Disease results from either functional harm, damage, or a combination of these factors. Colonization pertains to the presence of a fungus growing in nonviable tissue. A contaminant is simply a fungus that is present, usually as conidia or hyphal fragments. Depending on the situation, infection, colonization, and contamination represent reference points on a continuum of potential host–fungus interactions.
Saprophytic fungi may be involved in disease in at least three manners. The first and most important is the ever-increasing list of opportunistic infections. They occur in the setting of immunologic compromise, either due to human immunodeficiency virus (HIV) infection or associated with the use of cytotoxins and steroids to treat neoplasia (particularly hematologic neoplasia), to prevent organ transplant rejection, and to treat certain collagen vascular and arthritic diseases. Often, the degree of immunosuppression dictates which fungal opportunists will be involved. The most common agents are Candida albicans and C. tropicalis, which come from mucosal reservoirs in the patient. The lungs are the entry portal for the common extrinsic opportunists Aspergillus species, Zygomycetes, and Cryptococcus neoformans. In addition to these commonly encountered opportunistic fungi, infections caused by many other fungi have been documented. Fusarium species in some cancer patient populations and Pseudallescheria boydii are prominent entries in the list of fungal opportunists; reports of their involvement in infection appear almost monthly. The list of opportunistic fungal pathogens is so long that two new disease entities have been defined: phaeohyphomycosis and hyalohyphomycosis.
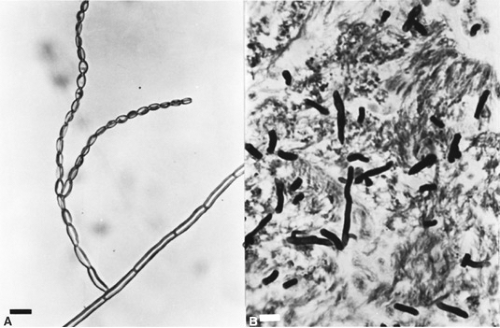
The various types of phaeohyphomycosis are caused by phaeoid or dematiaceous fungi (the latter having constitutive melanin pigment in their cell walls) associated with soil and plants. Nearly 110 such agents have been documented, the majority since 1975. The microscopic and histologic appearance of one such agent, Cladophialophora bantiana, is shown in Fig. 215.1; the clinical disease is shown in Fig. 215.2. The forms of hyalohyphomycosis are caused by fungi that lack melanin pigment in their cell walls. More than 70 species have been confirmed as capable of causing infection. Phaeohyphomycosis tends to be more necrotic and erosive, and generally the causative agents do not respond to treatment with amphotericin B. In contrast, in hyalohyphomycosis, thrombotic phenomena with local invasion are common, and most organisms
have some sensitivity to amphotericin B. Both these entities can be subdivided into superficial, cutaneous, subcutaneous, systemic, and allergic, depending on tissue involvement and mechanism of disease development.
have some sensitivity to amphotericin B. Both these entities can be subdivided into superficial, cutaneous, subcutaneous, systemic, and allergic, depending on tissue involvement and mechanism of disease development.
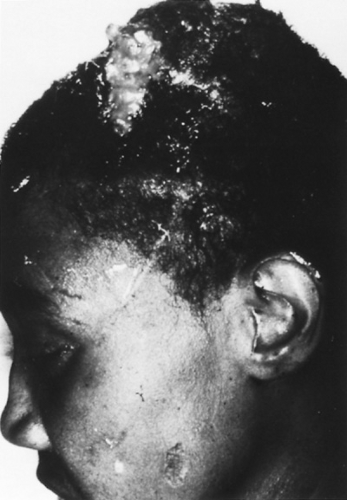 FIGURE 215.2. Cerebral phaeohyphomycosis. The organism Cladophialophora bantiana was acquired by inhalation and disseminated to the brain, producing a massive tumor that eroded through the scalp. |
A barrier break is the second circumstance by which soil organisms may gain entrance to protected organ systems. This factor is a predisposition much less common than is immunosuppression, but such cases do occur. Barrier breaks may be introduced by surgery, indwelling catheters, the use of nonsterile material in drug abuse, intrathecal injections, and ambulatory hemodialysis, among others. Reports have cited a mushroom growing on a mitral valve after open-heart surgery, of Scopulariopsis granulomas in the lung after the injection of crude opium, and of Aspergillus meningitis following the intrathecal use of contaminated steroids. Often, the same fungi involved in these diseases are found in the opportunistic categories.
Colonization of injured or debilitated tissue is the third category. Commonly, this predisposing situation is cited in reports of Alternaria invading the nose after a submucous resection, Fusarium colonizing burned skin, Zygomycetes infecting the injured foot of a diabetic, and Pseudallescheria infecting the knee after a soccer injury or auto accident. Many patients are not immunosuppressed. If viable tissue is invaded, the colonization becomes infection.
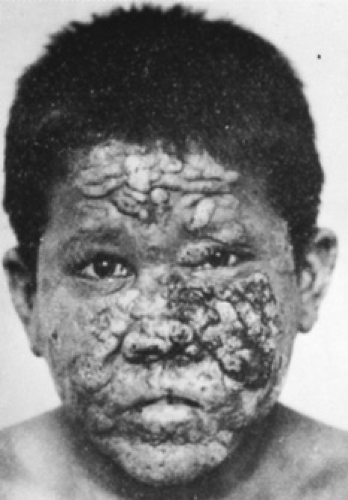
These three categories—opportunism, barrier break, and colonization of debilitated tissue—account for the vast majority of miscellaneous mycoses. Occasionally, a case occurs in which no predisposing factor is detected. The patient appears otherwise healthy. Reported cases include Mycocentrospora acerina (a plant pathogen) infection of the face (Fig. 215.3), Aureobasidium pullulans (a soil saprophyte) involvement of the skin, progressive ulceration of the dermis by the water mold Saksenaea vasiformis, and granuloma of the lung containing the insect pathogen Beauveria bassiana.
HYALOHYPHOMYCOSIS
Approximately 1 agents have been recovered from the clinical entity known as hyalohyphomycosis. Most affected patients have been immunosuppressed. The agents gain entrance through the lungs, sinuses, indwelling catheters, dialysis tubing, and gastrointestinal tract. In tissue, all produce branching, septate, hyaline mycelia that are morphologically identical to that seen in invasive aspergillosis.
Allescheria boydii was described by Shear in 1922 as the causative agent of a mycetoma of the foot of a patient who lived in western Texas. The fungus was a homothallic (self-fertile) organism that produced sexual spores in a fruiting body. In addition, it produced at least two types of asexual conidia. The asexual phase already had been noted from various types of fungal diseases since 1899; in 1911, it was given the name Monosporium apiospermum. In 1944, Emmons realized that the sexual phase and conidia-producing phase were in fact one species. At present, the organism is known as Pseudallescheria boydii during the sexual stage and as Scedosporium apiospermum as the asexual conidia-producing form. P. boydii is common in sewage, stagnant water, and barnyard manure.
Since the 1890s, P. boydii has been identified as the etiologic agent of a variety of clinical syndromes, including ear infections in children. Usually, these infections followed a primary bacterial otitis externa and were chronic, and treating them was difficult. By far, the most common form of P. boydii infection is white-grain mycetoma, which is seen particularly in the world’s temperate zones. In addition, sometimes P. boydii produces fungus balls in old pulmonary cavities, similar to Aspergillus, and can cause both allergic bronchopulmonary hyalohyphomycosis and invasive pulmonary infection also similar to Aspergillus (Fig. 215.4). Other syndromes caused by this species include osteomyelitis, arthritis, surgical wound infection, sinusitis, keratitis, endophthalmitis, infection of vascular grafts, endocarditis, and disseminated infection.
The rarity of P. boydii infections began to change in the 1960s and 1970s, when the generalized use of high-dose steroids, cytotoxins, and other immunosuppressive agents created a large susceptible patient population. Opportunistic infections of all types became common, and among these were fungus infections. Candida, Aspergillus, Cryptococcus, and Zygomycetes were encountered (in that order). Most affected patients had hematologic neoplasia and, if treatment resulted in neutropenia, fungus infections often quickly developed. In the 1980s, an additional population of organ transplant recipients increased the pool of susceptible individuals. The once rare infections caused by P. boydii have increased to the point that this organism now ranks just below the Zygomycetes in number of cases produced.
In the setting of immunosuppression, P. boydii infections usually begin in the lungs, with almost any organ becoming involved after hematogenous dissemination. A variety of changes in the lungs are visible on roentgenography, ranging from nonspecific infiltrates to cavitating lesions. Infections progress rapidly, producing necrotizing pneumonitis, pulmonary abscesses, and nodular infarcts. Dissemination occurs to the brain and other organs. Histologically, the branched, regularly septate mycelium seen in tissue sections is identical to those seen in disseminated aspergillosis.
Another form of P. boydii infection results from the aspiration of contaminated water or sludge or from the introduction of material into immunocompetent patients by injury or accident. In the first form, more than a dozen cases have been reported in children who aspirated stagnant water, sewer pond sludge, pig manure, or sheep dip, or who nearly drowned in swimming pools. An aspiration pneumonia results, and the organism disseminates. Often, the course is protracted. Football injuries, bruises from soccer playing, and motorcycle and automobile accidents have caused lacerations that later were followed by chronic osteomyelitis caused by P. boydii. Osteomyelitis in this setting usually does not respond to antifungal therapy, and amputation may be necessary in some cases.
Generally, P. boydii resists treatment with amphotericin B and many imidazole drugs. Ketoconazole and miconazole have been successful in a few cases, although the parenteral form of miconazole is not available in the United States (Table 215.1). Voriconazole, a new imidazole, and terbinafine, an allylamine derivative, have shown promise as therapeutic agents for this fungus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree