CHAPTER 14 Microfracture
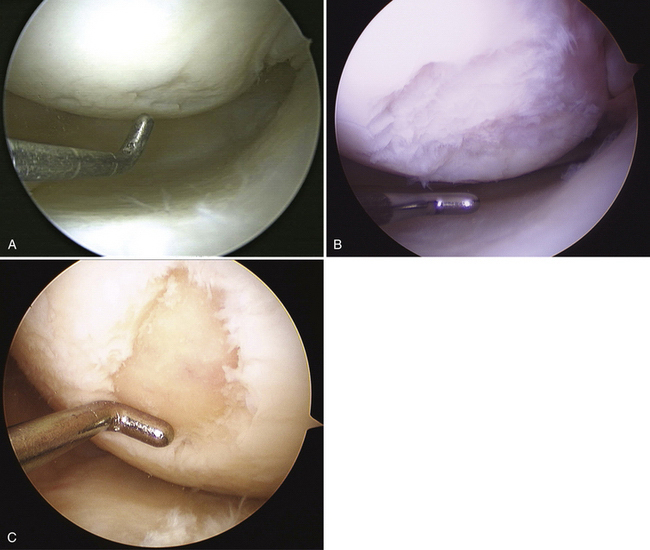
Articular cartilage lesions are a commonly encountered condition seen during arthroscopic surgery (Fig. 14-1)1,2 and may be encountered incidentally in spite of improvements with magnetic resonance imaging (MRI). The limited repair capabilities of articular cartilage chondrocytes and the avascular nature of hyaline cartilage do not allow for any intrinsic healing capacity of full-thickness articular cartilage lesions that do not violate the subchondral bone.3,4 Different techniques have been used in an attempt to create reparative or transfer cartilaginous tissue into the defect with the goal of improved pain relief, activity tolerance, and avoidance of long-term degenerative changes to the lesion or adjacent cartilage.5 Multiple procedures have been used in the past several decades to promote reparative “regenerate” cartilaginous tissue ingrowth into the lesion.6–8
In the mid-1980s, Rodrigo and colleagues9 and Steadman and associates10 adapted the Pridie drilling technique by using an awl to create perforations within the subchondral bone layer. The technique was refined with the knowledge of the importance of the removal of the calcified cartilage layer, overlying the subchondral bone, to improve adherence to the subchondral bone.11
After penetration of the subchondral bone, bleeding from the bone marrow creates a fibrin clot within the defect. Marrow-derived pluripotential cells progressively differentiate into fibroblasts, osteoblasts, chondroblasts, and chondrocytes, without contribution from the adjacent articular cartilage. Shapiro and coworkers12 have demonstrated after cellular labeling via autoradiography that the repopulation is mediated entirely by the proliferation and differentiation of mesenchymal cells from the bone marrow. The collagen matrix formed did not integrate into the adjacent native cartilage and this weak link is theorized to be the potential source of long-term repair tissue degradation. The repair tissue fibrocartilage is also primarily composed of type I collagen, which has decreased resilience and stiffness compared with the type II collagen seen in normal hyaline cartilage.
The risk of articular cartilage defect progression to degenerative arthritis is multifactorial and defect size may play a significant role in this process. A critical size of articular defect that is potentially damaging to adjacent cartilage is not known; the literature shows wide variability in defect size for which treatment had been indicated. Guettler and colleagues13 have evaluated pressure readings from the peripheral rim of articular defects created at various sizes, ranging from 5 to 20 mm. Rim stress concentration was seen in defects of 10 mm and larger, suggesting this as a critical size defect, which may increase the risk of progressive deterioration of the adjacent cartilage.
TREATMENT
Indications and Contraindications
Indications
Microfracture treatment is ideally indicated as a first-line treatment of well-contained Outerbridge grade III or IV cartilage lesions less than 4 cm2. Treatment of larger lesions has been described but most studies included lesions with a mean defect size of less than 4 cm2.14 Unstable chondral flaps that extend to the subchondral bone layer are also lesions amenable to this technique.
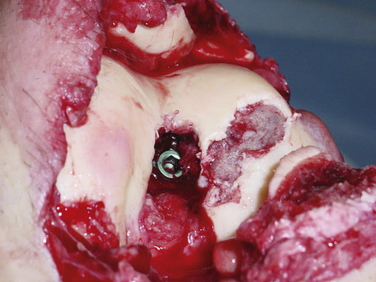
Secondary to its technical ease and limited surgical morbidity, microfracture marrow stimulation can be applied to cartilage lesions detected preoperatively by MRI or those found incidentally at the time of arthroscopic evaluation. We have also treated acute traumatic lesions in the polytrauma patient in the appropriate clinical setting (Fig. 14-2).
Patients sustaining acute, traumatic, chondral full-thickness lesions are best treated as soon as is practical logistically because duration of symptoms of less than 12 months has been associated with better postoperative outcome scores and improved microscopic repair cartilage grading.15–17 Patients with chronic or degenerative lesions are initially treated with nonsurgical measures, including activity modification, oral anti-inflammatory medication, physical therapy, dietary modifications, and weight loss, as well as potential articular injections such as corticosteroids or hyaluronic acid. Patients who fail nonoperative treatment are potential candidates for microfracture.
Age younger than 40 years, has been associated with better clinical outcome scores and better repair cartilage fills.18,19 Increased body mass index has been demonstrated to be universally correlated with poor outcomes after microfracture treatment, with worst outcomes seen in those with a body mass index (BMI) more than 30 kg/m2.15
These indications need to be compared with the limited knowledge we have concerning the natural history of nonoperative treatment of articular cartilage lesions and the lack of control groups in trials evaluating operative treatment of these lesions.20 Some studies have demonstrated no difference in outcome for nonoperative management of these lesions compared with operative interventions.21,22 Patient selection also needs to be considered in view of the intensive postoperative rehabilitation program that is usually prescribed following microfracture surgery. Weight-bearing limitations in the postoperative rehabilitation program result in the interruption of normal lifestyle and often induce disuse muscle atrophy.
Contraindications
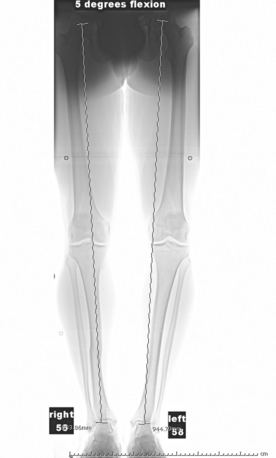
Axial malalignment of more than 5 degrees for lesions in the tibiofemoral joint is an indication for simultaneous or staged corrective osteotomy (Fig. 14-3). Not only is joint malalignment detrimental to normal articular cartilage, but uncorrected malalignment can result in significantly increased contact pressure in the affected compartment, resulting in increased load and shear on the treated lesions.23
High-grade ligamentous instability will also result in increased shear force across the treated lesion and is a contraindication to microfracture treatment unless surgically treated simultaneously.23
Patient Evaluation Prior to Treatment
The evaluation of the patient for treatment of an articular cartilage lesion requires the assessment of a large number of variables. The history of present illness should include assessment of the mechanism of injury, with documentation of the acuity and cause of the lesion. Patients who have undergone previous nonsurgical and/or surgical treatment need to be questioned about the types of treatments and their response, because these will play a role in future treatment options. Patients previously having undergone surgical treatment should have a careful evaluation of their preoperative imaging, arthroscopic images, and operative reports. Articular cartilage lesions that have undergone previous treatment that violates the subchondral bone layer are unlikely to gain improvement via microfracture.
Physical examination includes documentation of height and weight for determination of BMI. Standing alignment and gait assessment should be included in the examination. The varus thrust in stance phase or varus recurvatum gait would be consistent with a double- and triple-varus knee,24 respectively, and should be considered contraindications to treatment of a medial compartment articular cartilage lesion, unless addressed prior to considering microfracture. Ligamentous integrity of the cruciate and collateral ligaments must be determined, because a deficiency results in abnormal knee translation and resultant excessive shear force on the articular cartilage. Assessment of residual loss of motion or muscular weakness is important because these may play a role in abnormal joint forces and resultant symptomatology. Previous incisions should be evaluated for neuroma formation.
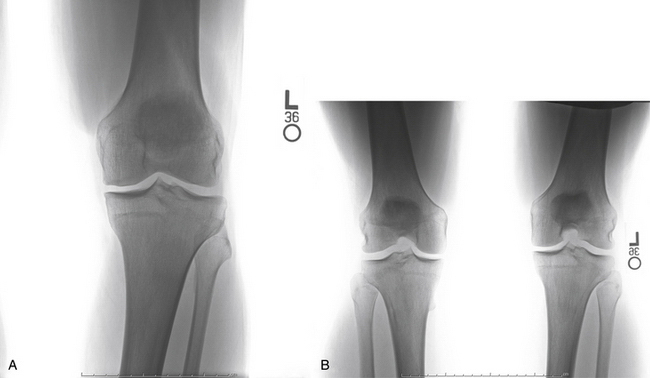
Our radiologic evaluation includes weight-bearing images, including anteroposterior (AP), lateral, Rosenberg (posteroanterior taken at 45-degree flexion), and axial patellar views (Laurin) (Fig. 14-4). A full-length hip to ankle weight-bearing alignment film should also be obtained in the patient undergoing assessment of an articular cartilage lesion. The weight-bearing line (WBL) is measured by drawing a straight line from the center of the femoral head to the center of the tibiotalar joint.25 The mechanical axis is measured by the angle created from a line drawn from the center of the femoral head to the midpoint between the tibial spines in comparison to a line from the center of the tibial spines to the center of the tibiotalar joint. Simultaneous or staged corrective osteotomy should be considered for patients who have greater than 5 degrees of tibiofemoral varus or valgus, based on the involved compartment. Patellofemoral alignment is evaluated via the axial patellar view and trochlear and patellar morphology seen by MRI.
MRI is useful for the preoperative assessment of the size, depth, and location of the articular cartilage lesion.20,26,27 Several acquisition protocols have been recommended by the International Cartilage Repair Society (ICRS).28 Spoiled gradient-recalled echo (SPGR) and fast spin-echo (FSE) techniques are the most accurate and widely used.29,30 New pulse sequences and image acquisition methods are being investigated for improved diagnosis of articular cartilage abnormalities, disease progression, and biologic response to treatment strategies.29–31 The status of the ipsilateral meniscus is important to assess, particularly in a patient having undergone prior surgical treatment and partial meniscectomy. Adjacent and opposing articular surfaces also need to be carefully evaluated. The subchondral and metaphyseal bone are assessed for cyst formation, bone loss, or cavitation. Loss of osseous integrity or sphericity may play a role in impaired fibrocartilaginous ingrowth. The peripheral aspect of the lesion is also evaluated to determine whether there is containment of the lesion, because this is important to the success of microfracture treatment.