CHAPTER 13 Degenerative Arthritis
Arthroscopic débridement for OA of the knee was initially reported by Burman and colleagues in 1934.1 In 1941, Magnuson introduced the term “joint debridement.”2 During and after World War II, arthroscopy waned and the open Magnuson procedure, consisting of total synovectomy, osteophyte resection, cruciate ligament excision (if torn), and patellectomy became the treatment of choice for arthritis of the knee until the resurgence of arthroscopy in the early 1970s.3
ANATOMY
In 1743, William Hunter stated, “From Hippocrates to the present age, it is universally allowed that ulcerated cartilage is a troublesome thing and that once destroyed it is not repaired.”4 In 1849, Leidy confirmed this principle, stating that “a rupture of cartilage fragments is never united and that articular cartilage lacks regenerative power and the joint becomes filled with tough fibrous tissue.”5 As noted by Mankin, superficial lacerations of cartilage neither heal nor progress to more serious disorders if they are small lesions, but deep lacerations may be clearly visible years after injury.6 When the subchondral bone is thus disrupted, interosseous blood vessels expose bone matrix growth factors, causing fibrin clot formation. Inflammation introduces new cells into the cartilage defect and these cells proliferate and begin matrix repair. The matrix of articular cartilage is a hyperhydrated tissue, with estimates of water content ranging as high as 80%; it contains type I collagen consisting of two alpha chains and one alpha-2 chain. It is this type I collagen that is formed when fibrous tissue regenerates when attempting to form normal hyaline articular cartilage.7 Furthermore, mature repair tissue has a relatively low proteoglycan concentration. These reparative cells do not produce tissue with the unique composition, structure, and biochemical properties of normal articular cartilage.8 After cartilage injury or during the progression of osteoarthritis, some chondrocytes proliferate but do not migrate through the matrix to enter the site of tissue injury. The repair tissue matrix, which is usually formed by undifferentiated cells containing primarily type I collagen, thus cannot restore normal articular cartilage properties. These reparative cells fail to organize the molecules that they produce to create a strong cohesive structure such as that of articular cartilage; rather, they produce other types of molecules that may interfere with the assembly of the cartilage matrix.
These alterations compromise the ability of cartilage to survive and function in the highly stressed mechanical environment found in load-bearing joints and may lead to further cartilage degeneration and osteoarthritis. Disruption of collagen cross-linking causes cartilage to lose its intrinsic tensile stiffness, strength, and shear stiffness, and this loss of proteoglycans and increased water content compromises its compressive and permeability properties.9,10 A number of treatments have been attempted to stimulate repair or reformation of the articular surface of the knee joint. Arthroscopically, these treatments include marrow stimulation procedures, débridement and shaving of fibrillated cartilage, and joint lavage.
TREATMENT
Arthroscopic Technique
Marrow Stimulation Procedures
The concept of drilling through eburnated bone to stimulate reparative cartilage formation was originally described by Pridie in 1959 (Fig. 13-1).11 Laboratory animal studies attempted to confirm these findings. Akeson and associates12 drilled the subchondral bone of dog femoral heads and Mitchell and Shepard13 drilled holes into the subchondral bone of rabbit knee joints. Both groups noted deterioration of the repair tissue within 1 year.
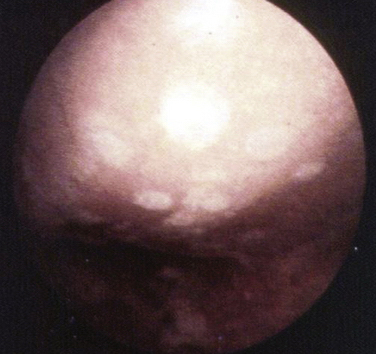
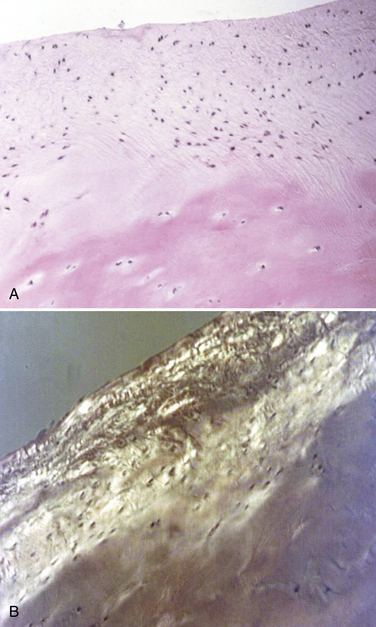
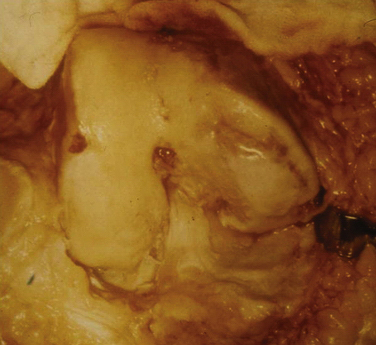
These experiments were the first to show that perforation of subchondral bone could stimulate repair of large areas of joint surface with fibrocartilaginous tissue, but the repair tissue lacked the proteoglycan concentration found in normal cartilage that was noted previously. Abrasion arthroplasty of grade IV eburnated chondral lesions using an arthroscopic burr to expose interosseus bleeding was introduced by Johnson14 in 1981 (Fig. 13-2). The resulting hemorrhagic exudate formed a fibrin clot, allowing for fibrous repair tissue formation over the eburnated bone (Fig. 13-3). Only one of eight biopsy specimens showed any type II collagen typical of hyaline cartilage at the time of arthroscopic review and biopsy, and the remainder had types I and III collagen. In a series of patients at our institution who had abrasion arthroplasty, at 5-year follow-up examinations, 15 had been converted to total knee replacement (TKR) and biopsies were obtained at the time of TKR. All patients had fibrocartilage and type I collagen on their biopsy specimens (Fig. 13-4). In this series of 126 patients who were treated for unicompartmental OA with abrasion arthroplasty plus débridement or arthroscopic débridement alone, only 51% had good to excellent results with abrasion arthroplasty at 5-year follow-up; 66% had good to excellent results with arthroscopic débridement alone.15 Coventry and Bowman16 have noted that formation of hyaline-like cartilage occurs in the unloaded medial compartment of several patients after valgus upper tibial osteotomy (Fig. 13-5). This finding was confirmed arthroscopically by Fujisawa and coworkers17 12 to 18 months after upper tibial osteotomies, which implies that regeneration of reparative cartilage can occur secondary to unloading of bone alone without additional surgery. Because adult articular cartilage does not heal and the regenerative tissue is not hyaline cartilage but fibrocartilage, it lacks normal amounts of proteoglycan. Thus, effective methods of repairing significant cartilage defects must provide cells that can migrate, proliferate, and differentiate in the chondral sites and produce and maintain a cartilage matrix. Furthermore, there must be a mechanical and biologic environment that promotes synthesis, assembly, and maintenance of articular cartilage matrix. This regenerative fibrocartilage has a difficult time transferring compressive loads and, therefore, cannot be expected to survive in an abnormal mechanical environment.7–10
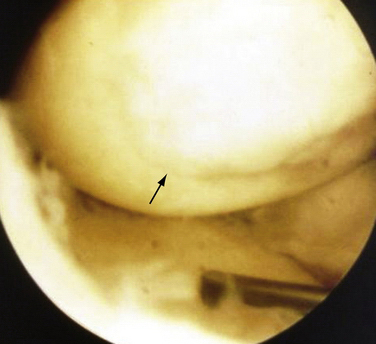
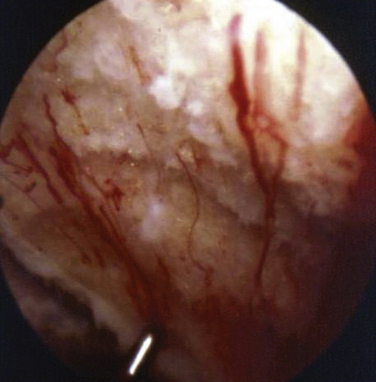
FIGURE 13-3 Arthroscopic view of a patient 4 years after abrasion arthroplasty showing resurfacing with fibrocartilage.
Microfracture
Blevens and colleagues18 have recommended the microfracture (MF) technique, in which they use an arthroscopic awl to create multiple perforations into the subchondral bone. They reported 266 patients between 1985 and 1990, with 3.7-year follow-up, using a grading system similar to that of the Outerbridge classification. After chondral surface débridement, the bone is perforated to a depth of 3 to 4 mm using an awl and the holes are placed approximately 4 to 5 mm apart in a full-thickness lesion with exposed subchondral bone (Fig. 13-6). Blood should be seen emanating from the microfracture holes after perforation is complete. A postoperative rehabilitation program was used to provide motion without applying high load stress to the treated chondral defect. Repeat arthroscopies were performed in 80 patients. In most chondral defects, subchondral bone was covered with cartilage of varying quality, and the term hyaline-like
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree