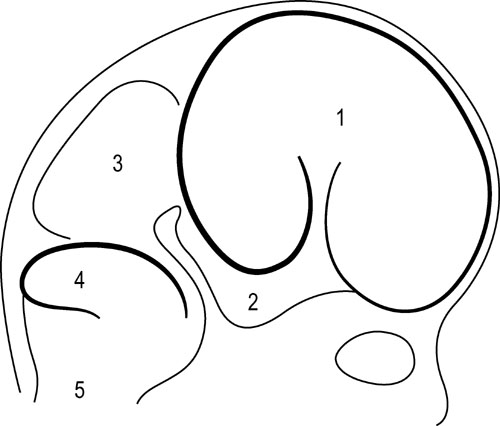
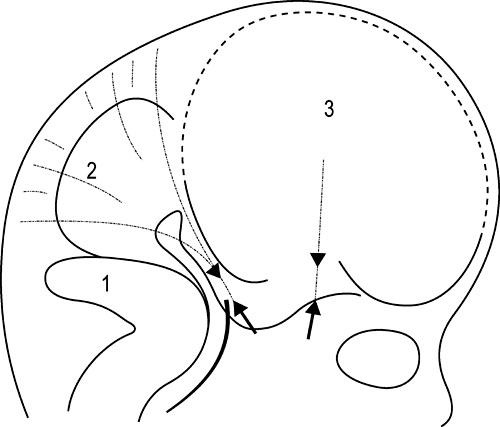
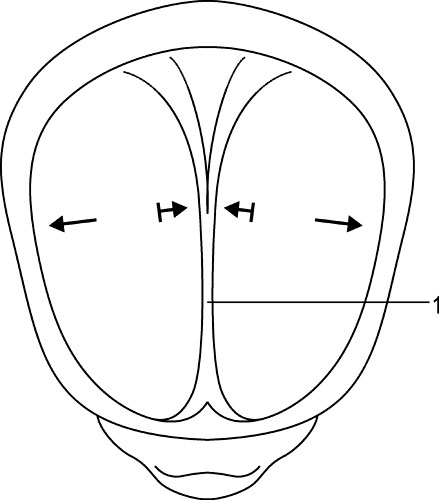
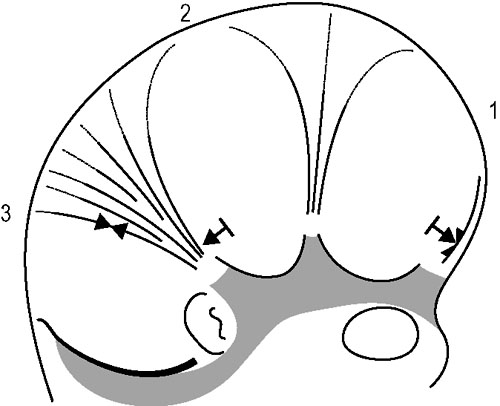
1.9 Membranous structures within the cranial bowl and intraspinal space Blechschmidt (1973, 1978) advises that connective tissue forms according to the environmental forces at hand (Figs 1.9.1–1.9.3). The well-vascularized nervous system creates different biodynamic fields that lead to tensile stress in the back of the developing embryonic spine and to compression in the front of it. In the course of that development, the base of the skull beneath the brain is flattened and compressed and is formed as cartilaginous pre-structures (Fig. 1.9.4) . The cranial roof is formed out of skin under tensile growth-stress and the bony structures are created by dermal ossification of flattened and tensed membranous connective tissue. Because of that development, the outer dura mater is a strongly anchored to the inside of the cranial bones. With increasing excentric growth of the cerebrum, the tensile resistance of the antibasal and laterodorsal cranial walls increases gradually, so that the brain regions bend away from each other. The brain fissures develop, in which falx and tentorium become denser. Falx cerebri and tentorium cerebella develop by compression of mesenchymal tissue between the brain hemispheres, respectively between the cerebrum and cerebellum during embryonic brain growth. Fig. 1.9.1 • Influence of the brain on the development of the cranium according to Blechschmidt. Schema of brain portions of an embryo of 28 mm. 1: Cerebrum, 2: diencephalon, 3: midbrain, 4: cerebellum, 5: medulla oblongata. Modified from Blechschmidt, 1978, with permission. Fig. 1.9.2 • Influence of the brain on the formation of the cranium according to Blechschmdt. Expansion of the developing brain induces formation of a thick fascial membrane (dura) between the cerebellum and cerebrum, as well as of a smaller membrane between the frontal and temporal lobes of the cerebrum. Convergent arrows: developmental push of the brain is met by tensional resistance of dural membranes. 1: Cerebellum, 2: midbrain, 3: right cerebral hemisphere. Embryo size approx. 29 mm. Modified from Blechschmidt, 1978, with permission. Fig. 1.9.3 • View of right and left dural membranes in the frontal area. Left and right dural membranes are shown. Arrows: developmental push of both cerebral hemispheres. 1: Falx cerebri as very tough fascial membrane. Embryo size approx. 29 mm. Modified from Blechschmidt, 1978, with permission. Fig. 1.9.4 • Lateral view of dural membranes. The densation field at the basis of the dural membranes leads to later development of the cartilaginous cranial base (pointed area). Convergent arrows: dural membrane capable of resisting tension. Smaller arrows: developmental expansion of brain. Embryo size approx. 29 mm. Modified from Blechschmidt, 1978, with permission. The dura mater consists of dense, uneven, very strong connective tissue with a lot of collagenous fibers. It is very tight and not permeable to the CSF. A special layer of flat fibroblastic cells without extracellular collagen and extracellular space can be found at the transition between the dura and arachnoidea (Haines et al. 1993). This dural borderline can be divided into periostal dura and meningeal dura. There is no epidural space like that in the spine. The dura mater continues as the epineurium of efferent nerves that leave the skull. The skin of the scalp and the dura are connected by emissary veins, which may be under enormous tension. In the region of the ethmoid bone cells, the tegmentum and sinus sigmoideus, the dura is very flat. The inner layer (dura meningealis) is weaker in its structure than the outer dura layer or the arachnoidea (Haines et al. 1993). The dura of an adult can resist stronger forces than that of a newborn (Dragoi 1995). According to Arbuckle (1994), this fibrous structure enables the intracranial dura and the intraspinal dura to transmit different forces. These “pathways for transmission of forces” find their way by the fibrous structures of the dura, the so-called “stress-fibers”. Arbuckle defines the following groups: horizontal, vertical, transversal, and circular. The direction of fibers in the dura mater cranialis can possibly be traced back to the results of mechanical forces during embryonic development, when collagenous fibers are brought into line by stress forces (Hamann et al. 1998). Between the dura periostale and dura meningeale there are some other important structures apart from the venous blood sinuses. • Endolymphatic bag: a sort of baglike tube, part of the ductus endolymphaticus, which is located at the rear wall of the petrous bone between the two dura layers. • Meningeal arteries: terminal branches of the carotid arteries. • Sympathetic nerve fibers travel between the dural layers of intracranial vascular walls (coming from the superior cervical ganglion and the plexus caroticus), as do the sensitive fibers of the 5th and 10th cranial nerves and of the 1st and 2nd cervical nerves. • Trigeminal cave (Meckel’s cavity): a peculiar cavern of the dura for the ganglion of the 5th cranial nerve (also called trigeminal nerve ganglion, semilunar ganglion, or Gasserian ganglion), located at the front side of the apex of the petrous part of the temporal bone above the foramen lacerum. The membranes inside the cranium are connected anatomically as well as functionally and so affect each other. Due to their different positions and orientations they can be divided into four septa: the falx cerebri, tentorium cerebella, falx cerebelli, and diaphragma sellae. Collagenous fiber bundles of the falx cerebri and falx cerebella form arcs in the anterior, intermediate, and posterior regions that cross each other perpendicularly. In the course of growth, fiber organization modulates from a 45° angle to a 90° angle (Dragoi 1995). According to Delaire (1978), the horizontal system (tentorium cerebella and diaphragma sellae) acts as a clamp or tightener for the cranial base, while the vertical system (falx cerebri and falx cerebella) tightens the cranial vault. Delaire argues that the tension in the horizontal and vertical dural system is maintained and regulated by the continuous tone of the neck muscles and the sternocleidomastoid muscle, but this is controversial. According to Ferré et al. (1990), movement of the neck muscles can be transmitted via the aponeurotic part of the scalp. However, only very weak and secondary movement can be sensed there, even though the aponeurotic structure of the scalp is clearly movable, unlike the highly immobile falx cerebri and falx cerebelli. According to Sutherland (1939), tension in every part of the membranous system can have an influence at all other parts of that system due to the structural bond. The dural membranes safeguard the integrity of the cranium, especially in early childhood, by way of their attachment to the cranial bones, in case of exposure to force. Additionally, it is thought that involuntary “jointed” movements of similar cranial bones are regulated via synchronicity with the rhythm of the primary respiratory mechanism. Every difference in tension at one side of the membrane alters the complete unit and leads to a new balance. The tentorium cerebelli (“La Tente”, Winslow 1732) divides the cerebrum and cerebellum from each other and stretches over the cerebellum like a tent. Above the tentorium there are, apart from the cerebral hemispheres, the subcortical nuclei and the thalamus. Like the falx cerebri and falx cerebella, the tentorium starts at the straight sinus and is attached there, too. The tentorium is attached posteriorly at the inner occipital protuberance and at both sides at the transversal groins of the internal occipital bone, where it forms the transverse sinus. Sideways, it leads along the sinus across the parietomastoideal suture and attaches for a short distance with its superior layer to the inferior back edge of the parietal bone. Meanwhile, the inferior layer can be found as an attachment to the mastoid process of the temporal bone. This is an important place, because its attachments continue from there along the mastoid process and the superior edge of the petrous part of the temporal bone, where it encloses the superior petrosal sinus. The inferior lateral layers are attached to the clinoid processes of the sphenoid bone. The free internal borders of the tentorium continue in an anterior direction, cross over the anterior inferior layers, and are attached to the anterior clinoid processes of the minor wings of the sphenoid bone. Here, where the internal branches of the tentorium cross the outer branches, the abducent nerve can be found. Obviously, the abducent nerve can be disturbed by tentorium tension. A large oval opening, the tentorial incisura, is occupied anteriorly by the midbrain and the interpeduncular cistern, and posteriorly by the rounded end (or splenium) of the corpus callosum.
Embryonic growth dynamics of the dural membrane according to Blechschmidt
Intracranial membrane system
Dura mater (hard outer layer of the meningeal membranes)
Horizontal and vertical dural system
Musculoskeletal Key
Fastest Musculoskeletal Insight Engine









