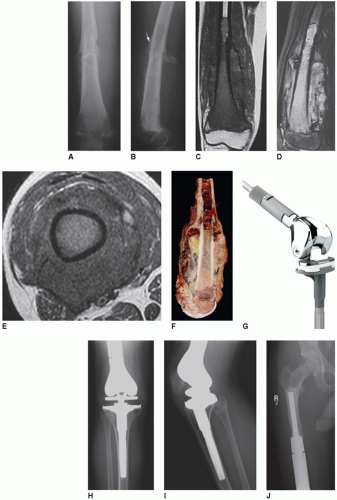
Megaprosthesis of the Distal Femur
Kristy L. Weber
The distal femur is a common site for primary malignant bone tumors and, therefore, it frequently requires resection and reconstruction in an orthopedic oncology practice. In addition, patients who require multiple revision surgeries after a routine total knee replacement (TKR) for degenerative arthritis may end up with substantial bone loss, necessitating a resection of the distal femur. Therefore, this chapter is relevant to both orthopedic oncologists and revision knee surgeons alike. The examples provided are primarily from patients with bone tumors, but the technique section should apply to both situations. For more detail related to reconstruction for non-neoplastic conditions, see chapter 15. Although reconstructive options after resection of the distal femur include an osteoarticular allograft, allograftprosthetic composite, and allograft arthrodesis, this chapter focuses on the use of a distal femoral megaprosthesis with rotational hinged knee replacement. The rationale for the modular rotational hinged knee design is that it provides stability in the anterior-posterior and varus-valgus planes while allowing rotation of the tibia on the femur. Theoretically, stress at the bone-cement interface is diffused by this rotational ability and decreases the risk of aseptic loosening. The goal of this type of reconstruction (as opposed to allograft or arthrodesis reconstruction) is to provide the patient a rigid, durable reconstruction that will allow immediate weight bearing (cemented stems).
INDICATIONS
The indications below do not distinguish between cemented and uncemented prosthetic designs. In general, the choice between these options is surgeon-dependent as both are reported in the literature with similar outcomes.
Patients who require resection of the distal femur for aggressive benign or malignant primary bone tumors (Figs. 16.1 and 16.2)
Patients who require resection of the distal femur for malignant soft tissue sarcomas that invade the bone
Patients with extensive metastatic disease to the distal femur with destruction of the condyles and no good option for stable nonprosthetic fixation
Patients with bony tumor recurrence or aseptic loosening of a distal femoral megaprosthesis
Patients with severe distal femoral bone loss combined with ligamentous instability
Patients with an acute periprosthetic femur fracture or nonunion that cannot be stabilized or healed with a stemmed revision prosthesis, allograft struts, or internal fixation
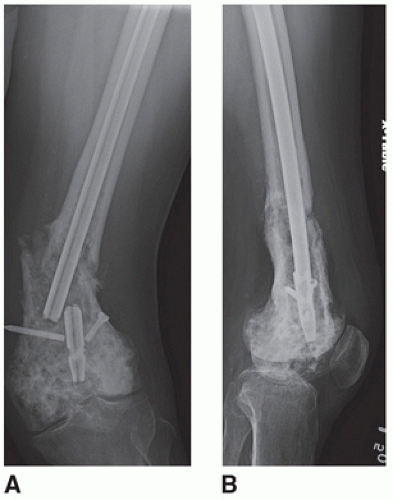
Patients with a history of high dose radiation to the distal thigh or femur and a subsequent fracture (Fig. 16.3)
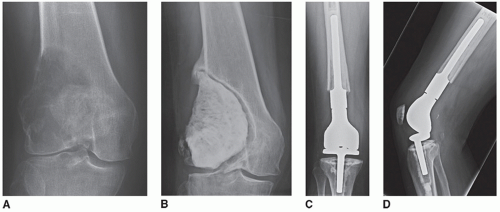
Patients with failed fixation or nonunion of a distal femoral fracture (Fig. 16.4)
Patients with severe osteoporosis and a complex supracondylar or intracondylar femur fracture
CONTRAINDICATIONS
Patients in whom an adequate surgical margin cannot be obtained with resection and reconstruction. In these cases, an amputation should be considered.
Patients with significant comorbidities such that they would not survive the procedure (i.e., cardiac, pulmonary)
Patients with an infected knee joint, history of knee infection or systemic sepsis (although there are options short of amputation to reimplant a megaprosthesis after clearing the infection with intravenous antibiotics and an antibiotic-impregnated cement spacer within the knee joint)
Patients receiving chemotherapy who have not had a reasonable recovery of their absolute neutrophil count prior to surgery (author prefers ANC > 1,500)
Patients whose malignant bone tumor or skip metastasis extends to within 130 mm of the proximal end of the femur as this does not allow stable fixation of the distal femoral stem. In this case a total femoral replacement can be considered.
Patients whose distal femoral sarcoma has progressed while on chemotherapy. These patients are usually not candidates for limb salvage surgery.
Tumor involvement of the femoral neurovascular bundle or sciatic nerve. This would be a relative contraindication as these structures can be resected and bypassed, but the overall function of the leg is decreased.
Tumor involvement of the surrounding quadriceps to a significant degree that requires complete removal of the compartment is a relative contraindication. A free, innervated latissimus dorsi flap can be used in these situations to regain some knee extension.
Patients who are extremely young, as the prosthesis is often custom-made and will require multiple revisions as well as limb/prosthetic lengthening. This is a relative contraindication. The author generally recommends rotationplasty or amputation to patients <10 years old as this is usually definitive surgery and requires no restriction from physical activities. The author acknowledges that there are expandable custom implants currently used in very young children for limb salvage procedures.
PREOPERATIVE PREPARATION
Patient Expectations
It is imperative to confirm the patient’s understanding of the goals and likely outcomes of the surgery prior to the procedure. The surgery is being done to provide local control of a malignant bone tumor or to salvage a prior failed reconstruction and should be agreed upon by the patient and surgeon (and family for children <18 years of age) as the most appropriate procedure. The patient will be expected to work diligently in the postoperative period to regain knee motion and quadriceps strength in order to walk as normally as possible. This will require regular exercise and, often, formal physical therapy. Depending on whether a cemented or uncemented prosthetic design is used, the patient may be restricted initially from placing full weight on the extremity. The patient needs to be aware of the long-term outcomes of distal femoral reconstruction with a megaprosthesis, which include aseptic loosening. The surgeon may suggest restrictions on impact loading activities to maximize the lifespan of the prosthesis, and this may affect a patient’s lifestyle or recreational activities.
History/Physical Examination
At the time of the preoperative consultation, an updated history and physical examination should be performed with careful attention to recent changes in signs and symptoms such as pain, neurologic status, fevers, fatigue, weight-bearing status, recent infection, and injury to the extremity. On physical examination, the entire extremity should be carefully assessed for knee passive and active range of motion, motor strength (especially quadriceps), deformity, sensation, leg length discrepancy, signs of an infected knee joint, abrasions to the extremity, ingrown toenails, or a rash. Prior incisions or open biopsy tracts should be documented, and it should be determined if this will influence the surgical approach. The author is not concerned about the presence of needle biopsy tracts and does not excise them during the procedure, but there are differing opinions on this point.
Radiographs
In the older, nononcologic patient, plain radiographs that include an appropriate amount of femur and tibia as well as a chest radiograph should be sufficient. Oncology patients should have new plain radiographs of the distal femur to include the knee and a postchemotherapy MRI scan to assess any change in tumor size. If the local tumor has progressed while on chemotherapy, the patient is likely no longer indicated for a limb salvage procedure. The MRI scan will also allow careful evaluation of the marrow extent of tumor so that the resection can be done with a clear margin. The status of the soft tissue and neurovascular bundle involvement is critical. If the tumor involves the neurovascular structures, an MR angiogram or standard angiogram may be warranted. Oncology patients should also be restaged for their overall disease with a new chest CT scan. Generally, these
patients have already had a bone scan to identify skip metastasis if present. Skip metastases in the femur are usually incorporated into the resection.
patients have already had a bone scan to identify skip metastasis if present. Skip metastases in the femur are usually incorporated into the resection.
Laboratory Tests
Patients should have laboratory tests prior to surgery that include a complete blood count, protime, prothrombin time (PT), electrolytes, and a type and screen/cross for blood products. The hemoglobin should be assessed to determine the potential need for packed red blood cells during the case. For oncology patients, the white cell count (including absolute neutrophil count) should be adequate to minimize the chance of postoperative infection. It is also important to check the calcium and magnesium as these can be low with chemotherapy. Adult patients for revision knee surgery with a megaprosthesis should have laboratory studies that are appropriate for their comorbidities (i.e., liver function tests, etc.). If there is any concern for infection in the native knee or prior knee reconstruction, a C-reactive protein, erythrocyte sedimentation rate, and joint aspiration should be performed.
Consultations
The anesthesia team should be consulted in advance to determine the method of anesthesia (regional, general, or combined). It is critical in oncology patients or nononcology patients with a significant valgus deformity at the knee to check the neurologic status of the limb postoperatively. For patients who have received Adriamycin, a preoperative echocardiogram may be requested by the anesthesia team. For patients over 50 years old, an ECG is often necessary. Additional preoperative clearance may be necessary from the Cardiology, Pulmonary, or General Medicine services as determined by the patient’s medical condition. In the oncology patient, a Plastic Surgery consultation should be obtained if a large soft tissue defect or loss of the quadriceps/extensor mechanism is anticipated as part of the tumor resection.
Equipment Needs
Templating for the anticipated prosthesis and stems is done on the preoperative radiographs. It is important to determine whether the femoral canal can accept the minimum standard diameter stem (usually 11 mm). If not, smaller stems need to be available. The length of the remaining proximal femur after resection should be determined, as 130 mm is necessary for standard length femoral stems. Additional equipment may be needed as follows:
Cemented versus uncemented femoral component
Polyethylene versus metal stemmed tibial component
Supplemental autograft or allograft (possible allograft struts for revision cases)
Patellar resurfacing component (The author does not resurface the patella in young oncology patients, but reserves this for older patients with arthritis affecting the patellofemoral joint).
Intraoperative fluoroscopy
Doppler to assess vascular supply to foot
Cement removal instruments for revision cases
Hardware removal instruments if internal fixation is present
Body exhaust system/hood (depending on surgeon preference)
TECHNIQUE
This technique is primarily geared toward placement of a cemented prosthesis (cemented femoral stem and polyethylene stemmed tibial component). When appropriate, alternative technical points related to uncemented designs are noted. The use of a polyethylene tibial component, as opposed to one with a metal baseplate and modular stems, requires that the polyethylene component be adequately supported by cortical bone around its periphery. In cases of poor quality proximal femoral bone in either primary or revision situations, an uncemented Compress system (Biomet, Inc.) may be indicated (1). This chapter is not focused on the placement or lengthening techniques for expandable prostheses in skeletally immature patients.
Setup
The patient is placed in the supine position on an operating table that can accommodate fluoroscopic views. Anesthesia is given. The author prefers that the patient not be paralyzed for the resection when close dissection is required around the neurovascular bundle. A urinary catheter is placed. The leg is prepped and draped in its entirety (including the foot) above the level of the anterior superior iliac spine to allow for placement of a sterile tourniquet if necessary. The author uses a sandbag taped across the operating table at the level of the knee to facilitate stabilizing the knee in an upright flexed position during the reconstruction. A blood transfusion may be necessary intraoperatively or postoperatively, especially if the patient has been on preoperative chemotherapy, so an appropriate type and cross should be confirmed.
Incision
Elevate the extremity and inflate the tourniquet. Standard incision options include anterior and lateral. However, the incision may need to be modified to accommodate open biopsy scars or prior knee incisions. Prior open biopsy incisions need to be removed by incorporation into the new incision so as to avoid potentially leaving residual viable tumor cells in the scar. The author finds the anterior incision to be the most versatile and easiest to use if the patient requires subsequent revision knee surgery. Start the incision proximal to the level of planned femoral resection and end just medial to the tibial tubercle. Protect the patellar tendon throughout the case, especially when the patella is everted laterally.
Resection
The finer points of tumor resection can be supplemented from other textbooks, as this book is focused on the reconstructive techniques. It is presumed that an intra-articular resection will be performed in the vast majority of cases. Briefly, the distal femur is dissected circumferentially to allow for a safe resection. The author cuts longitudinally along the rectus femoris tendon during the initial exposure, leaving a small medial cuff of the tendon to facilitate a tight extensor mechanism repair. The patella is everted laterally, and the joint and joint fluid are inspected. If there is extensive hemorrhage or soft tissue to suggest that the tumor has contaminated the knee joint, an extra-articular resection should be considered. The vastus intermedius is usually left covering the underlying bone tumor, although at times more of the quadriceps should be resected depending on the size, extent of soft tissue mass, and location of the tumor. The vastus medialis is retracted medially. The medial collateral ligament is transected just proximal to the medial meniscus. Medially, the origin of the gastrocnemius and insertion of the adductor muscles are released from the femur. This may be facilitated by flexing the knee. Care must be taken when dissecting along the adductor canal, as the femoral vessels are tethered close to the medial femur in this location. The femoral vein is the most medial structure at this level, and there may be multiple branches draining the tumor. These are ligated so that the main neurovascular bundle can be retracted posteriorly. The medial intermuscular septum separating the anterior and posterior compartments of the thigh is incised to allow dissection along the distal posterior femur into the popliteal fossa. Branches of the popliteal artery and vein leading to and from the femoral intercondylar notch are dissected and ligated, as the vessels are tethered to the femur in this location. The medial dissection continues proximal to the level of the planned bone transection (the author prefers to cut the femur at least 2 cm further than the most proximal location of disease noted on a preoperative T1-weighted MR image).
For the lateral dissection, the lateral quadriceps and majority of the rectus femoris muscles are retracted laterally along with the patella, which is everted when possible. The lateral patellofemoral ligaments are cut. The lateral collateral ligaments are transected just proximal to the lateral meniscus. The lateral intermuscular septum separating the anterior and posterior thigh compartments is identified and carefully dissected to allow entrance to the posterior compartment. The origin of the lateral head of the gastrocnemius muscle is released from the femur. Dissection continues proximal to the level of planned bone transection and circumferential subperiosteal dissection is done at this level. Mark the mid-anterior femoral cortex just proximal to the planned cut with an osteotome and marking pen for later rotational alignment during the reconstruction. Do this prior to cutting the cruciate ligaments or the true rotation may be disrupted. The author prefers to then cut the femur proximally with a power saw perpendicular to the femur while protecting the posterior soft tissues with a malleable retractor. Other surgeons may prefer to disarticulate the femur at the knee joint and transect the femur at the end of the dissection. At this time, a large curette is used to obtain a frozen section at the proximal femoral marrow margin. After the sample is removed, bone wax is placed over the end of the bone to prevent spillage of marrow contents. Bone wax is also placed on the end of the distal femoral segment to prevent spillage from the tumor side. A Kern or other large clamp is used to elevate the distal femoral fragment from its proximal end and the dissection continues posteriorly to free the remaining soft tissue attachments. Meanwhile the frozen section is being analyzed as opposed to sending the frozen section after the complete resection, which can cause a delay prior to reconstruction while the tourniquet is still inflated. The anterior cruciate ligament (ACL) is sharply divided from its tibial insertion. The posterior cruciate is divided either anteriorly or posteriorly. The posterior knee capsule is finally divided while protecting the popliteal vessels. At this point the specimen is removed from the operative table, and the resection length is carefully measured from the cut proximal end to the medial joint line. At the author’s institution, the musculoskeletal pathologist immediately cuts the distal femoral specimen coronally with a table saw so that the gross distance from the cut femoral end to the visible tumor can be measured. Along with the frozen section results, the surgeon uses this information to determine whether additional bone needs to be resected to allow for an adequate margin.
For patients with nononcologic indications for distal femoral resection, the femur can often be subperiosteally dissected to maintain the majority of the quadriceps. The remaining steps are similar. If a standard, unstemmed knee prosthesis is in place, the femur can be transected just proximal to the femoral component. The standard prosthetic distal femoral segment is 65 mm in length added to a length of 10 to 40 mm depending on whether a porous coated body segment is included with the stem; so this should be taken into account when planning the resection length. Use of the cement removal or cementless revision instruments is required when removing
a stemmed femoral prosthesis. Fluoroscopic guidance is used to minimize the possibility of penetrating the cortex during prosthesis or cement removal. A cortical window proximal to the end of the stemmed femoral component can facilitate its removal, although this requires that onlay strut grafts are cabled to the femur during the reconstruction or a longer stemmed revision is used to bypass this cortical defect and minimize a postoperative fracture. The author uses a sterile tourniquet unless the dissection extends too proximally in the thigh. It is ideal if the entire resection and reconstruction can be completed within a 2-hour tourniquet time. If not, the tourniquet should ideally stay inflated throughout the resection until the margins are clear. It can then be reinflated after being deflated for at least 30 minutes.
a stemmed femoral prosthesis. Fluoroscopic guidance is used to minimize the possibility of penetrating the cortex during prosthesis or cement removal. A cortical window proximal to the end of the stemmed femoral component can facilitate its removal, although this requires that onlay strut grafts are cabled to the femur during the reconstruction or a longer stemmed revision is used to bypass this cortical defect and minimize a postoperative fracture. The author uses a sterile tourniquet unless the dissection extends too proximally in the thigh. It is ideal if the entire resection and reconstruction can be completed within a 2-hour tourniquet time. If not, the tourniquet should ideally stay inflated throughout the resection until the margins are clear. It can then be reinflated after being deflated for at least 30 minutes.
Prosthetic Reconstruction
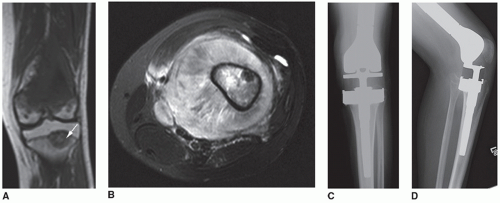
The operative team changes gowns, gloves, instruments, light handles, suction, electrocautery, and superficial drapes when performing a prosthetic reconstruction after malignant tumor resection. For a prosthetic revision case or for indications of massive bone loss or fracture without malignancy, this step is not necessary. The wound is irrigated with pulsed lavage saline with or without antibiotics and hemostasis is obtained (Fig. 16.5).
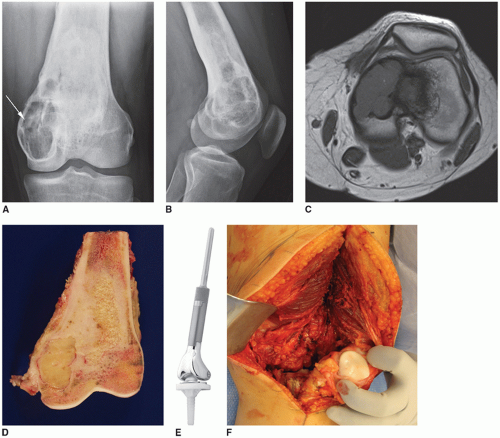 FIGURE 16.5 AP (A) and lateral (B) radiographs of an 18-year-old woman with a recurrent grade 1 fibrosarcoma of the left distal femur (see arrow). She was treated for a presumed benign tumor with curettage and bone grafting 1 year ago. C: T1-weighted axial MRI showing the endosteal cortical erosions from the tumor. There is minimal soft tissue extension medially. D: A coronal view of the gross resected specimen. E: An example of a distal femoral megaprosthesis with a polyethylene tibial component. F: View of the defect after tumor resection with the patella exposed showing no degenerative changes. G: Sharp removal of the menisci from the tibial plateau. H: Flexible reaming of the proximal femur slightly further than the length of the prosthetic stem (for cemented stems). I: Making the tibial cut using the appropriate jigs. J: Using both an intramedullary and extramedullary alignment guide to judge rotation of the tibial tray. K: Placement of the trial prosthesis to assess length and tension on the neurovascular structures. L: Injection of cement into the tibia after the punches and keel have been used. No cement restrictor is necessary as a bone plug is created with the tibial punches. M: It is important to identify where the keel of the polyethylene tibial component will be placed within the cement to assure appropriate rotation. N: Placement of the real polyethylene component with excess cement removed. O: Injection of cement into the femur that was previously irrigated and dried. P:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|