Chapter 151 Megaprostheses for Reconstruction Following Tumor Resection About the Knee
Rationale for Use of Megaprostheses
Options for reconstruction following resection of the distal femur or proximal tibia include the use of a megaprosthesis—an implant designed to replace the resected large bone segment—or the combined used of a structural allograft with a revision-type arthroplasty implant (allograft-prosthetic composite). In the past, a perceived advantage of an allograft-prosthetic composite was the potential for bone stock restoration. However, long-term studies have shown that little of the structural allograft actually becomes remodeled to viable bone9 and resorption of the allograft and fracture can occur over time.2 Allograft-prosthetic composites provide the potential for effective host tendon healing to allograft tendon, a critical advantage in reconstruction of the extensor mechanism following resection of the proximal tibia (see Chapter 150). Although new implant materials (e.g., trabecular metal) show great potential for effective healing of host tissue and tendon to the actual metal prosthesis, such implants have been designed and should be released to the orthopedic market in the near-future.27
Indications
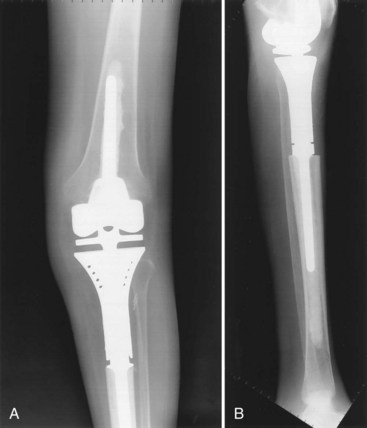
Following resection of the proximal tibia, a segmental modular rotating hinge megaprosthesis knee system is appropriate for patients who require postoperative radiation or have an extensor mechanism that cannot be reconstructed with an allograft-prosthetic composite (Fig. 151-1). Although chemotherapy can retard bone healing at the allograft-host bone junction, the administration of postoperative chemotherapy is not an absolute contraindication to the use of an allograft-prosthetic composite in the proximal tibia. If trabecular metal pads in the tibial tubercle region of a proximal tibial replacement megaprostheses prove effective in providing a solid method of attachment of the patellar tendon to the implant, I believe that the use of allograft-prosthetic composites for reconstruction of the proximal tibia will significantly diminish.
Methods of Fixation of Megaprostheses
Fixation of the intramedullary stems of the femoral and tibial components may be achieved with various methods. Both cemented and uncemented fixation have been shown to be effective.5,11,12,15,28 Cement fixation should be considered for patients with poor-quality bone and those who will require postoperative radiotherapy. Some surgeons favor the use of a hydroxyapatite coating on the intramedullary stem component of megaprostheses to promote fixation of the uncemented stem.4,21,25,26
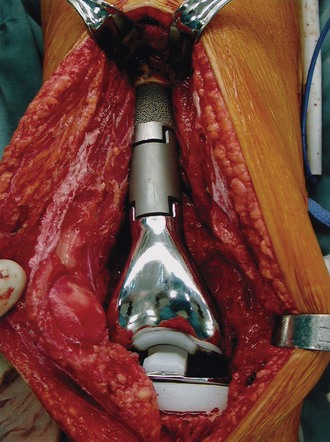
With press-fit or cement stems, implant systems will typically have an option of a porous-coated collar on the intramedullary stem component (Fig. 151-2). This design feature is to encourage the formation of extracortical bone bridging; this is bone that forms between the host bone and this collared portion of the implant (Fig. 151-3). Bone graft material is placed in this region prior to wound closure to promote bone bridging. Ward and colleagues36 have postulated that extracortical bridging between the host bone and implant with bone or soft tissue may retard osteolysis by preventing implant debris–containing synovial fluid from contacting the bone-implant or bone-cement interface (Fig. 151-4).

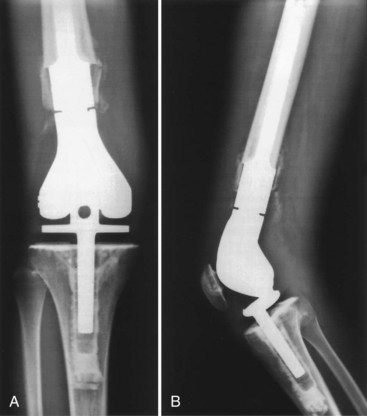
Figure 151-3 Close-up radiograph of host bone implant junction illustrating extracortical bone bridging.
The degree of development of extracortical bone bridging has been variable. In the distal femur, extracortical bridging develops more commonly posteriorly and along the compression side of the femur.18,20 Chao and associates6 have found that the total percentage of the length of the porous-coated region that was covered with bone formation in the distal femur and knee was 75% ± 31% in 15 implants, with follow-up of 2 to 21 years. The formation of this extracortical bone bridging stabilizes by 2 years following surgery. They also found that the prevalence of stem loosening is very low, suggesting that extracortical bone bridging improves long-term fixation. Kawai and coworkers19 have noted an association between extracortical bone bridging and higher limb function scores.
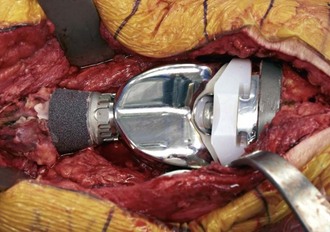
Clinical data have suggested that the presence of extracortical bone bridging is helpful to long-term success. However, the bone callus of the extracortical bone bridging may not actually grow into the porous coating of the implant. Lucent lines between the extracortical bone and porous surface may be observed.18 Furthermore, histologic analysis of five retrieved tumor megaprostheses has shown no bone ingrowth into the porous-coated segment of the implant, despite radiographs of these implants suggesting bone ingrowth into the porous-coated segment.30 Rather, transmitted light microscopy has shown fibrous tissue between the extracortical bone and porous coating. Nonetheless, the presence of this extracortical bone–fibrous tissue may increase prosthetic stability. With the application of new implant materials that promote the ingrowth of bone more effectively (e.g., trabecular metal), true extracortical bone bridging of bone ingrowth into the porous surface may occur (Fig. 151-5).
A relatively new method of achieving bone ingrowth fixation of a megaprosthesis is compressive osseointegration. Developed by Dr. James O. Johnston, a porous-coated titanium surface with a conical section is mounted transverse to the axis of the femur. Compression of the implant against host bone occurs through Belleville spring washers tightened by a bolt over an intramedullary traction bar. Retrieval data from 12 patients with Compress implants undergoing revision surgery for infection, periprosthetic fracture, or local tumor recurrence have shown that only 2 patients with infection demonstrated loosening at the bone-prosthesis interface without evidence of osteonecrosis at an average of 3.3 years postimplantation.22 In a comparison of uncemented femoral megaprosthesis fixation, the prosthetic survival rates at 5 years were comparable between 50 patients with intramedullary uncemented stem (85%) and 41 patients with Compress fixation (88%).10
Surgical Technique
Distal Femoral Replacement Arthroplasty
Although the degree of bone and soft tissue resection is dictated by the location of tumor, the extent of quadriceps removal influences postoperative function. In patients who had resection of the distal femur for tumor and reconstruction with a distal femoral replacement arthroplasty, resection of the vastus lateralis and vastus intermedius (and preservation of the vastus medialis) resulted in a more physiologic gait and knee-loading pattern than those seen in patients with resection of the vastus medialis (and preservation of the vastus lateralis).1 The importance of this data is in placement of the biopsy; if the surgeon has a choice, the preferred position of the biopsy should be lateral to allow preservation of the vastus medialis.
The level of the femoral osteotomy is marked and the vastus intermedius and any additional muscle to be transected at this level are then cut. The underlying vessels are protected during the osteotomy. The marrow at the proximal osteotomy margin is sent for frozen section analysis to confirm a negative margin. The proximal aspect of the resection specimen (distal aspect of femoral osteotomy level) is then retracted anteriorly, medially, or laterally to facilitate transection of the remaining soft tissues. The middle geniculate branch is identified and ligated. The popliteal vessels are easily identified and protected. The heads of the medial and lateral gastrocnemius muscles are detached, again leaving a cuff of tissue on the specimen. The knee capsule and collateral and cruciate ligaments are transected; this may occur after the osteotomy to facilitate mobilization of the distal femur. Negative margins are confirmed on frozen section analysis. After resection of the distal femur, the proximal tibia is prepared with a perpendicular bone cut as in a standard knee arthroplasty. The proximal tibia is prepared for the stem portion of the tibial component. The femoral canal is prepared for a cemented, press-fit, or composite fixation application. The patella may or may not be resurfaced, depending on the status of the articular cartilage.7 Trial components are placed to assess for limb length, soft tissue tension, and proper patellar tracking. After the final implant is inserted, the prostheses should be completely covered with soft tissue. If inadequate muscle remains, a medial gastrocnemius muscle flap should be considered. The implant should not be left in a subcutaneous position. A deep drain should be placed and perioperative antibiotics administered. Wound healing takes a priority over early knee motion. Typically, most patients gain excellent flexion; active extension is dependent on the quality of remaining quadriceps.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree