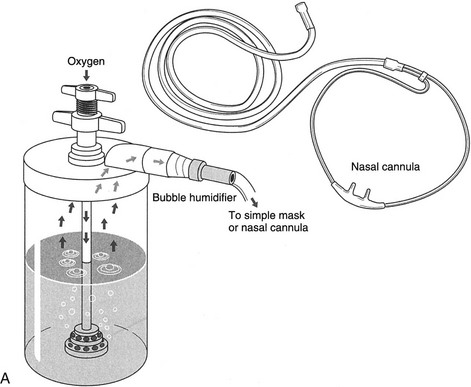
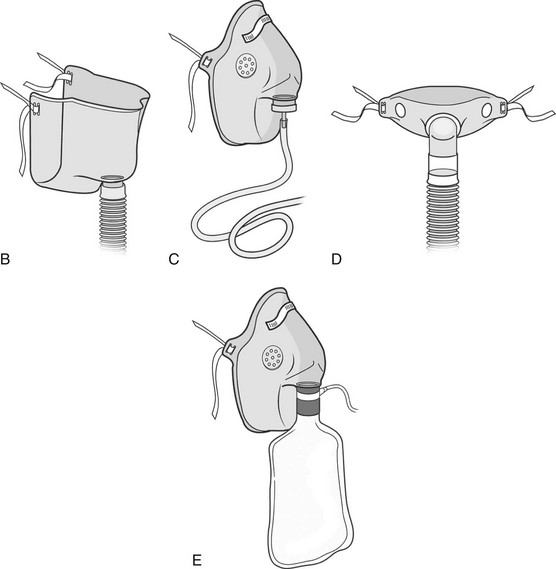
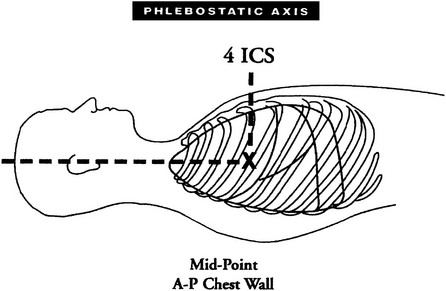
Chapter 18 The objectives of this chapter are to provide the following: 1 Describe the various types of medical-surgical equipment commonly used in the acute care setting, including oxygen (O2) therapy and noninvasive and invasive monitoring and management devices 2 Provide a framework for the safe use of such equipment during physical therapy intervention The acute care setting is multifactorial in nature and applies to many body systems. For this reason, specific practice patterns are not delineated in this chapter. Please refer to Appendix A for a complete list of the preferred practice patterns in order to best delineate the most applicable practice pattern for a given diagnosis. The general indication for O2 therapy is hypoxemia. Hypoxemia is considered to be present when the arterial oxyhemoglobin saturation (SaO2) is less than 90%, corresponding to an arterial blood O2 partial pressure (PaO2) of less than 60 mm Hg.1 Refer to Table 4-6 for the relation between O2 saturation as measured by pulse oximetry (SpO2) and PaO2 and to Figure 4-7 for the oxyhemoglobin dissociation curve. Other indications for O2 therapy are severe trauma, shock, acute myocardial infarction, surgery, or carbon monoxide or cyanide poisoning.1,2 The goal of O2 therapy is to prevent or reverse hypoxemia by increasing the PaO2, thereby improving tissue oxygenation, decreasing work of breathing, and decreasing myocardial work.3 O2 moves across the alveolar-capillary membrane by diffusion, the physiologic mechanism by which gas moves across a membrane from a region of higher to lower pressure, and is driven by the partial pressure gradient of O2 between alveolar air (PaO2) and pulmonary capillary blood. To improve diffusion, a rise in PaO2 can be attained by increasing the fraction of inspired O2 (FiO2) with supplemental O2.4 Supplemental O2 is delivered by variable-performance (Table 18-1) or fixed-performance (Table 18-2) systems. Each cannula or mask is designed to provide a range of FiO2. Variable-performance systems are not intended to meet the total inspiratory requirements of the patient and should not be used if a specific FiO2 is required. The actual FiO2 for a given flow rate in a variable system is dependent on a patient’s tidal volume and respiratory rate and the type, fit, and placement of the cannula or mask. If a specific FiO2 is required, then a fixed-performance system is indicated. Fixed-performance systems deliver a specific FiO2 despite the patient’s respiratory rate and pattern.2 TABLE 18-1 Variable-Performance Oxygen Delivery for Spontaneously Breathing Adults* FiO2, Fraction of inspired oxygen; lpm, liters per minute; O2, oxygen; RA, room air. *Listed from least to most oxygen support. Data from Cairo JM: Administering medical gases: regulators, flowmeters, and controlling devices. In Cairo JM, Pilbeam SP, editors: Mosby’s respiratory care equipment, ed 8, St Louis, 2004, Mosby, pp 60-88; Arata L, Farris L: Oxygen delivery devices. In George-Gay B, Chernicky CC, editors: Clinical medical surgical nursing, Philadelphia, 2002, Saunders, pp 126-127; Christopher KL, Schwartz MD: Transtracheal oxygen therapy, Chest 139(2):435-440, 2011. TABLE 18-2 Fixed-Performance Oxygen Delivery Data from Cairo JM: Administering medical gases: regulators, flowmeters, and controlling devices. In Cairo JM, Pilbeam SP, editors: Mosby’s respiratory care equipment, ed 8, St Louis, 2004, Mosby, pp 60-88; Heuer AJ, Scanlon CL: Medical gas therapy. In Wilkins RL, Stoller JK, Scanlon CL, editors: Egan’s fundamentals of respiratory care, ed 8, St Louis, 2003, Mosby. FIGURE 18-1 A, Nasal cannula with humidification. B, Open face mask or tent. C, Closed face mask. D, Tracheostomy mask or collar. E, Non-rebreather mask. (A, Redrawn from Kersten LD, editor: Comprehensive respiratory nursing: a decision-making approach, Philadelphia, 1989, Saunders. In Hillegass E, Sadowsky HS: Essentials of cardiopulmonary physical therapy, ed 2, Philadelphia, 2002, Saunders. B to E, From Dewit SC: Fundamental concepts and skills for nursing, ed 4, St Louis, 2014, Saunders.) FIGURE 18-2 Air entrainment mask or Venturi mask. (From Dewit SC: Fundamental concepts and skills for nursing, ed 4, St Louis, 2014, Saunders.) O2 delivery devices with masks or reservoirs allow O2 to collect about the nose and mouth during exhalation, which increases the availability of O2 during inhalation. As the storage capacity of the mask or reservoir is increased, the FiO2 for a given flow rate is also increased.2 A patient with chronic obstructive pulmonary disease (COPD) who has chronic carbon dioxide retention may become desensitized to the respiratory stimulant effects of carbon dioxide. In these patients, ventilation is driven by means of a reflex ventilatory response to a decrease in Pao2 and in theory, providing supplemental O2 may lead to a reduction in the hypoxic peripheral chemoreceptor ventilatory drive. The hypoxic drive is not usually suppressed until the PaO2 increases to greater than or equal to 60 mm Hg.5 Potential respiratory depression or hypercapnia should never contraindicate oxygen therapy in the case of hypoxemia, however.6 If acute respiratory failure occurs in the patient with COPD, other support measures such as noninvasive positive-pressure ventilation or invasive mechanical ventilation can be used.7 • Note that a green label designates the O2 supply on hospital walls. A similar gauge supplies pressurized air that is designated by a yellow label. • The FiO2 for a given system is dependent on its proper fit and application. Ensure that all connections are intact, that the O2 is flowing as indicated, and that the cannula or mask is properly positioned. • Provide extra lengths of O2 extension tubing if functional mobility will occur farther than 5 or 6 feet from the bedside (i.e., the wall O2 source). Ensure that the plastic adaptor between two lengths of oxygen supply tubing is secure. • Ensure that portable O2 tanks are turned on and have sufficient levels of O2 before use. Have backup tanks available. • Observe masks for the accumulation of mucus or clogging. Clear or change the cannula or mask if needed. • Monitor the patient’s skin for potential breakdown due to pressure from the cannula or mask. Provide appropriate padding (such as foam ear protectors) without interfering with the fit of the cannula or mask. • Significant supplemental O2 requirements usually indicate a respiratory compromise, which in turn may indicate the need to modify or defer physical therapy intervention. • Observe the patient for clinical signs of hypoxemia: shortness of breath, use of accessory muscles of breathing, confusion, pallor, or cyanosis. • Document the type and amount of supplemental O2 used during physical therapy intervention. This includes different amounts of FiO2 for rest and exertion or for a recovery period after exercise. • Oxygen is typically titrated (increased or decreased) according to physician prescription or according to a hospital protocol. The physical therapist should only titrate oxygen if an order to do so exists or if the situation is clinically appropriate. Communicate any changes in FiO2 to the nurse or respiratory therapist. • There may be specific guidelines set forth by third-party payers for the qualification of home oxygen. For example, Medicare requires documentation of oxygen saturation levels within 48 hours of durable equipment delivery with a requirement of a resting room air SaO2 less than or equal to 88%, a room air SaO2 less than or equal to 88% with exercise, and documented improvement of hypoxemia during ambulation with oxygen.8 Monitoring hemodynamic events provides information about the adequacy of a patient’s circulation, perfusion, and oxygenation of the tissues and organ systems. The objective of hemodynamic monitoring is to ensure optimal tissue perfusion and oxygen delivery while maintaining adequate mean arterial blood pressure.9 Many measurements of cardiac and intravascular pressures and volumes are interpreted to direct a therapeutic plan of care. Ideally, hemodynamic monitoring is accurate and reproducible, is as uninvasive as possible, and minimizes harm to the patient.9 Hemodynamic monitoring can be accomplished using noninvasive (Table 18-3) or invasive (Table 18-4) methods. TABLE 18-3 Noninvasive Medical Monitoring Data from Albertson B: Vital signs. In Craven R, Hirnle C, Jensen S, editors: Fundamentals of nursing: human health and function, ed 7, Philadelphia, 2013, Wolters Kluwer/Lippincott Williams & Wilkins Health, pp 331-335; Callahan JM: Pulse oximetry in emergency medicine, Emerg Med Clin North Am 26(4):869-879, 2008; Wheaton Franciscan Healthcare: Self-learning telemetry monitoring: applying and maintaining electrodes. http://www.wfhealthcare.org. Accessed July 3, 2012. TABLE 18-4 *PAOP (formerly called pulmonary capillary wedge pressure or PAWP). Data from Lough ME: Cardiovascular diagnostic procedures. In Urden LD, Stacy KM, Lough ME, editors: Critical care nursing: diagnosis and management, ed 6, St Louis, 2010, Mosby; Dirks JL: Cardiovascular therapeutic management. In Urden LD, Stacy KM, Lough ME, editors: Critical care nursing: diagnosis and management, ed 6, St Louis, 2010, Mosby. FIGURE 18-4 A, Arterial line tracing from different sites. B, Pulmonary artery (PA) catheter (four-lumen model) in a branch of a PA with the balloon inflated; the pulmonary capillary wedge pressure (PCWP) reflects left atrial pressure (LAP). LA, Left atrium; LV, left ventricle; RV, right ventricle. (A, From Yentis SM, Hirsh NP, Smith GB, editors: Anaesthesia and intensive care A-Z. An encyclopedia of principles and practice, ed 2, Oxford, UK, 2000, Butterworth-Heinemann, p 45. B, From Kersten LD, editor: Comprehensive respiratory nursing: a decision-making approach, Philadelphia, 1989, Saunders, p 758.) Noninvasive, or indirect, hemodynamic monitoring provides physiologic information without the risks of invasive monitoring and can be used in many settings. Invasive, or direct, measurements are obtained by penetrating the skin and inserting a cannula or catheter into a blood vessel, chamber of the heart, or both. The cannula or catheter is attached to a monitoring system, which consists of a transducer, amplifier, and oscilloscope for the display of the vascular waveforms and pressure measurements.10 Direct monitoring can provide continuous, accurate data; however, thrombosis, infections, air embolisms, and trauma are potential complications.11 During invasive hemodynamic monitoring, the level of the right atrium is the standard zero reference point and is identified by the phlebostatic axis—the intersection of the midaxillary line and the fourth intercostal space (Figure 18-3).10 The nurse will “level” the system using a carpenter’s level or laser-light level to align the patient’s phlebostatic axis with the transducer. Repositioning the patient may artificially alter waveforms by applying pressure to the catheter, shifting the catheter or stopcock, or shifting the phlebostatic axis relative to the transducer.10 The transducer is releveled when clinically indicated. Raising the level of the phlebostatic axis relative to the transducer gives false high readings; lowering the phlebostatic axis gives false low readings.10 FIGURE 18-3 The phlebostatic axis at the intersection of the fourth intercostal space (ICS) and the midpoint of the anterior (A) and posterior (P) chest wall. (Courtesy Edwards Lifesciences LLC.) Intracranial pressure (ICP) and cerebral perfusion pressure (CPP) may be measured in a variety of ways, depending on how urgently ICP values are needed and the patient’s neurologic or hemodynamic stability. The purpose of ICP monitoring is the maintenance of normal CPP and the early identification of increased ICP before the occurrence of secondary cerebral damage.12 Refer to Intracranial and Cerebral Perfusion Pressure in Chapter 6 for a description of these terms, Table 6-28 for a description of the early and late signs of increased ICP, and Table 6-29 for a list of treatment options to decrease ICP. Table 18-5 describes the different types of ICP monitors. For some ICP monitors, such as the intraventricular catheter, the sensor and the transducer must be level. Often, the zero point for the transducer is at the tragus, or the top of the ear. A normal ICP waveform has a triphasic sinusoidal waveform and should correspond to heart rate. TABLE 18-5 Intracranial Pressure (ICP) Monitors Data from Smeltzer SC, Bare BG, Hinkle JL, et al: Management of patients with neurologic dysfunction. In Smeltzer, SC, Bare, BG, Hinkle, JL, et al, editors: Brunner & Suddarth’s textbook of medical-surgical nursing, ed 11, Philadelphia, 2007, Lippincott Williams & Wilkins; Baumann JJ: Neurologic clinical assessment and diagnostic procedures. In Urden LD, Stacy KM, Lough ME, editors: Critical care nursing: diagnosis and management, ed 6, St Louis, 2010, Mosby, pp 718-719. • As with hemodynamic monitoring, be aware of the ICP value and the corresponding waveform on the monitor. The waveform may change shape (plateau wave) if cerebral hypoxia or ischemia occurs.13 • Momentary elevations in ICP will normally occur. A sustained elevation in ICP longer than 5 minutes is of concern13 and should be reported to the nurse. • Patients with elevated ICP are often positioned with the head of the bed at 30 degrees, which maximizes venous blood flow from the brain to help decrease ICP.13 Therefore be aware that lowering the head of the bed may increase ICP. Other positions that increase ICP are the Trendelenburg position, lateral neck flexion, and extreme hip flexion. • Additional conditions that increase ICP are the Valsalva maneuver, noxious stimuli, coughing, pain, stress, and frequent arousal from sleep.13 Multimodal neuromonitoring, or the simultaneous use of multiple types of invasive and noninvasive modalities, are used to care for patients with traumatic brain injury (TBI).14 This concept of brain monitoring includes common tests and measures combined with newer technologies. The goal is to prevent secondary ischemic and hypoxic injury from changes in brain metabolism after TBI.14 An overview of these monitoring technologies, which are typically used in larger hospitals that treat a significant population of patients with neurotrauma or after neurosurgery, are listed in Table 18-6. TABLE 18-6 Multimodal Brain Monitoring Technologies Data from Cecil S et al: Traumatic brain injury advanced multimodal neuromonitoring from theory to clinical practice, Crit Care Nurse 31(2):25-36, 2011. Various lines, tubes, catheters, and access devices make up the wide variety of medical-surgical equipment used in the acute care setting. In general, these devices may be peripheral or central, for short-term or long-term use, and inserted or applied at the bedside in a special procedure (e.g., under fluoroscopic guidance) or in the operating room. Table 18-715–23 describes the medical-surgical management devices most commonly encountered in the acute care setting. TABLE 18-7 Medical-Surgical Management Devices* *Listed in alphabetical order. Data from Urden LD, Stacy KM, Lough ME, editors: Critical care nursing: diagnosis and management, ed 6, St Louis, 2010, Mosby; Burchell PL, Powers KA: Focus on central venous pressure monitoring in an acute care setting, Nursing (Dec):39-43, 2011; Domke MN: Get a positive outcome from negative pressure, Nursing Made Incredibly Easy! (Jan/Feb):20-29, 2010; American Association of Neuroscience Nurses: Reference series for clinical practice. Care of the patient with a lumbar drain, ed 2, pp 1-15. http://www.aann.org. Accessed July 5, 2012; Craven R, Hirnle C, Jensen S, editors: Fundamentals of nursing: human health and function, ed 7, Philadelphia, 2013, Wolters Kluwer/Lippincott Williams & Wilkins Health; Fink J: Aerosol drug therapy. In Wilkins RL, Stoller JK, Scanlon CL, editors: Egan’s fundamentals of respiratory care, ed 8, St Louis, 2003, Mosby, pp 775-780; Minkler MA: What are those tubes for? What you need to know about central venous access devices, EMS Mag 37(5):46, 48, 50 passim, 2008; McEwen DR: Wound healing, dressings and drains. In Rothcock JC, editor: Alexander’s care of the patient in surgery, ed 14, St Louis, 2011, Mosby, pp 265-266; Ferrar-Hoffman DL, Krizman SJ: Neurosurgery. In Rothcock JC, editor: Alexander’s care of the patient in surgery, ed 14, St Louis, 2011, Mosby, pp 866-867; Simmons KF, Scanlon CL: Airway management. In Wilkins RL, Stoller JK, Scanlon CL, editors: Egan’s fundamentals of respiratory care, ed 8, St Louis, 2003, Mosby, p 654. FIGURE 18-5 Tubing attachment to three-chamber collection unit. (From Phillips N: Berry and Kohn’s operating room technique, ed 12, St Louis, 2013, Mosby.) • Before entering a patient’s room, review the medical record, particularly new orders, recent progress notes, and test results. Review graphic sheets for vital signs, noting trends or variations from the norms. • Note whether any particular precautions protecting the patient or the caregiver from specific pathogens are in place (e.g., contact precautions). Refer to Table 13-3 for a summary of infection prevention precautions. • Practice standard precautions. The likelihood of encountering bodily fluids is increased in the acute care setting, especially in the ICU. • Discuss your planned intervention with the nurse. Scheduled procedures may take precedence over this intervention, or it may coordinate well with another planned procedure. • On entering the patient’s room, take inventory. Observe the patient’s appearance and position. Systematically observe the patient, and verify the presence of all documented lines. Develop a consistent method of surveying the room: left to right, or top of bed to bottom of bed, to ensure that all lines and equipment are observed and considered in your treatment plan. Take note of all readings on the monitors before intervention. • Anticipate how your intervention may change the patient’s vital signs and how this will likely appear on the monitors. Be aware of which readings may change artificially owing to relative position change. • Using appropriate precautions, gently trace each line from the patient to its source. Ask for assistance, if needed, to untangle any lines or to free any lines that might be under the patient. • Ensure that there is no tension on each line before attempting to move the patient. • Never attempt to free a line that cannot be completely visualized! • Discuss with the nurse whether any lines can be removed or temporarily disconnected from the patient before your treatment. • Ask for appropriate assistance when mobilizing the patient. • Most invasive monitoring systems have two alarm controls: one to silence or discontinue the alarm for a few minutes, and another to disable or turn off the alarm. Do not silence an alarm without permission from the nurse! It is not recommended that the physical therapist disable an alarm. • If available and appropriate, use a portable telemetry monitor to maintain the continuity of the ECG when mobilizing a patient who has continuous ECG monitoring away from the bedside. • On completion of your treatment, ensure that all appropriate alarms are turned on and that the patient is positioned with the appropriate safety and communication measures in place. Notify the nurse of any change in the patient’s status. Appendix 18A: Mechanical Ventilation Mechanical ventilatory support provides positive pressure to inflate the lungs. Patients with acute illness, serious trauma, exacerbation of chronic illness, or progression of chronic illness may require mechanical ventilation.1
Medical-Surgical Equipment in the Acute Care Setting
Preferred Practice Patterns
Oxygen Therapy

Device/FiO2
Description
Air entrainment mask (Venti mask, Venturi mask)
FiO2 ≈ 24%-50%
Purpose:
Provides a specific concentration of supplemental O2.
Consists of:
A high-flow system with a closed face mask over the nose and mouth and a jet mixing device located at the base of the mask, which forces 100% O2 past an entrainment valve. The valve can be adjusted to entrain a specific percentage of RA to mix with the O2, allowing precise control of FiO2 (Figure 18-2).
Clinical implications:
The closed face mask interferes with coughing, talking, eating, and drinking and may be very drying and uncomfortable. Patients often remove the mask for these reasons.
Educate the patient on the importance of keeping the mask snug in place.
Humidification is not used when oxygen flow is < 4 lpm because humidification will interfere with O2 delivery.
Useful for patients with COPD who require a fixed precise FiO2.
BiPAP (Bilevel positive airway pressure)
FiO2 ≈ 21%-100%
Purpose:
Provides positive inspiratory and positive end expiratory pressure to decrease the work of breathing by reducing the airway pressure necessary to generate inspiration throughout the respiratory cycle. May be used to avoid intubation and mechanical ventilation in cases of acute respiratory failure. Often used in the hospital or home setting for the management of obstructive sleep apnea.
Consists of:
A closed mask with a clear soft gasket around its border, placed over the nose to fit tightly against the patient’s face. It is held firmly in place with straps around the top and back of the head.
Clinical implications:
BiPAP may deliver supplemental O2 at a specific concentration, or it may deliver RA.
Patients may feel claustrophobic owing to the tight fit of the mask. The equipment may be noisy; thus the therapist may need to speak loudly to communicate with the patient.
Air leaks can occur around the mask. Abrasions on the bridge of the nose can occur and may be prevented with a dressing that provides padding to the area without interfering with the tight fit of the mask. May also cause nasal congestion, nasal dryness, or rhinorrhea.
Depending on the patient’s oxygen requirements, BiPAP may be turned off, and alternate methods of O2 delivery may be used to allow the patient to participate in functional activities or an exercise program. The unit may also be placed on a portable intravenous pole or cart for this purpose.
T tube/piece
FiO2 ≈ 50%-80%
Purpose:
Provides a specific concentration of supplemental O2 to an intubated, spontaneously breathing patient while weaning from a ventilator.
Consists of:
A T-shaped large-bore plastic tube attached directly to an endotracheal or tracheostomy tube. Humidified O2 is delivered through one end of the T, and expired gas exits the other end. The tubing acts as a reservoir for O2, allowing a specific concentration of O2 to be delivered.
Clinical implications:
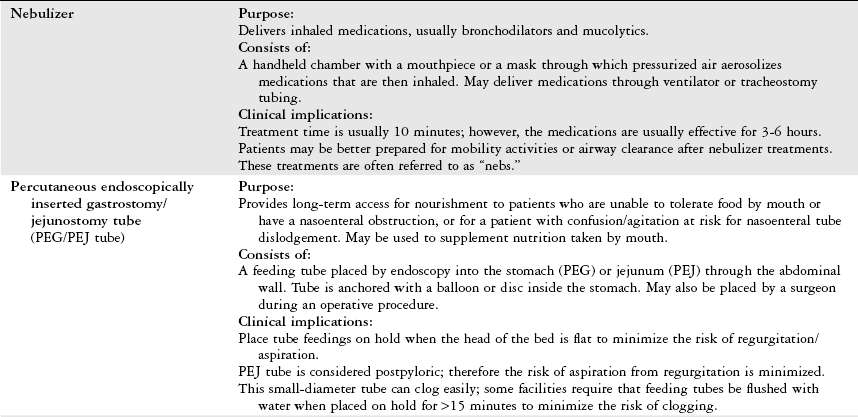
Tests the patient’s true ability to spontaneously breathe and allows for ventilatory muscle training.
Patients who are weaning from a ventilator can tire easily. Consult with the medical-surgical team to determine whether the patient will tolerate ventilator weaning (i.e., the use of a T piece) and physical therapy intervention simultaneously, or whether the patient would benefit from bronchopulmonary hygiene to facilitate weaning.
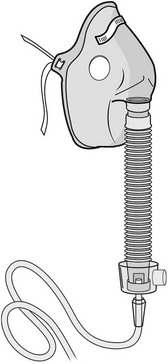
Physical Therapy Considerations
Hemodynamic Monitoring
Device
Description
BP cuff (sphygmomanometer)
Normal adult values: Systolic ≈ 100-140 mm Hg
Diastolic ≈ 60-90 mm Hg
Purpose:
Indirectly measures arterial blood pressure.
Consists of:
An inflatable bladder enclosed in a nondistensible cuff, attached to a pressure monitoring device. The device may be a manual aneroid manometer or an automatic oscillometric device. The cuff is typically placed 2.5 cm proximal to the antecubital space. If using a manual cuff, auscultate for Korotkoff sounds (refer to Table 3-7) with a stethoscope over an artery, usually the brachial artery. If using an automatic cuff, follow the manufacturer’s directions for operation.
Clinical implications:
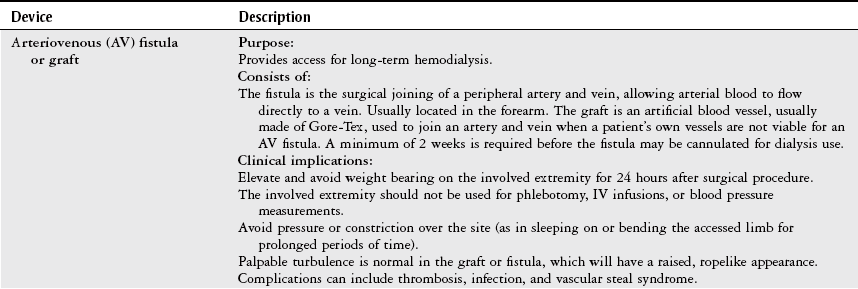
Do not use a BP cuff on an extremity with an arterial line, lymphedema, AV fistula or graft, or blood clot, or in an extremity ipsilateral to a mastectomy. Try to avoid measuring BP in an extremity with a peripheral or central intravenous line. Look for signs posted at the patient’s bedside stating whether the use of a BP cuff on a particular extremity is contraindicated.
Use an appropriately sized cuff. The cuff bladder should be no less than 80% of limb circumference. A cuff that is too small gives a falsely high reading, and a cuff that is too big gives a falsely low reading.
The cuff may be placed on the upper extremity distal to the elbow with auscultation of the radial artery.
Alternative sites for measurement in the lower extremity are proximal to the popliteal space with auscultation of the popliteal artery or proximal to the ankle with auscultation of the posterior tibial artery. These measurements are best taken with an automatic blood pressure cuff.
Avoid contact between stethoscope tubing and the cuff tubing to minimize extraneous sounds.
An automatic BP monitor may be used to take a blood pressure measurement if it is too difficult to hear Korotkoff sounds such as in the situation of hypotension. Automatic cuffs may also be helpful when a mean arterial pressure or serial blood pressures are needed, or when the therapist needs both hands (simultaneously) to guard a patient.
While an automatic BP cuff is inflating/deflating, be sure to keep the patient’s arm and hand still, and free from movement.
Be sure to document the location where a blood pressure measurement is taken in your note.
Telemetry (ECG)
Purpose:
Continuous monitoring of heart rate and rhythm and respiratory rate (see Table 3-9).
Consists of:
Five color-coded electrodes placed on the chest connected to a transmitter that converts the electrical currents from the heart into radio signals. Radio signals are picked up by antennae and transmitted to a central monitor at the nursing station and monitored at a distant site (telemetry). Twelve electrodes are used for a formal ECG.
Clinical implications:
Notify the nurse before physical therapy intervention, as many activities may alter the rate or rhythm or cause artifact (e.g., chest percussion).
If an electrode(s) becomes dislodged, reconnect it. One way to remember electrode placement is “white is right” (white electrode is placed on the right side of the chest superior and lateral to the right nipple), “snow over grass” (green electrode is placed below the white electrode on the anterolateral lower-right abdomen), and “smoke over fire” (black electrode is placed on the upper-left rib cage superior and lateral to the left nipple, and the red electrode is placed below the black one on the anterolateral left abdomen). The brown electrode is usually placed centrally in the fourth intercostal space.
Do not place electrodes over a pacemaker or defibrillator.
Artifact, or poor signal quality, can appear on telemetry because the strength and consistency of the electrical current are interrupted. Causes of artifact include patient movement, poor electrode contact with the skin, or manual techniques for bronchopulmonary hygiene. Patients on telemetry should be instructed to stay in the area monitored by telemetry antennas.
Watch the telemetry monitor to get a baseline heart rate and rhythm before physical therapy intervention and to ensure that the telemetry unit is actively connected. Telemetry may be put on hold temporarily for patient travel off the floor, or the batteries in an individual patient box may need replacement.
The telemetry box may fit in the pocket of a hospital gown or in a small pocket carrier that is placed around the patient’s neck or shoulder for exercise.
Telemetry boxes are usually equipped with a “record” button that when pressed will print a rhythm strip of the ECG at the time of an “event” (i.e., when the patient is symptomatic).
At some institutions, a designated nurse is stationed at the nursing desk to watch the main telemetry monitor for all patients. If indicated, check in with this nurse and request that the patient’s telemetry be carefully watched during physical therapy intervention.
Pulse oximeter
Normal SpO2 (at sea level) ≥ 93%-94%
Purpose:
A noninvasive method of measuring the percentage of hemoglobin saturated with O2 in arterial blood.
Consists of:
A probe (hard plastic clip or flexible strip) with an electro-optical sensor placed on a finger, toe, earlobe, forehead, or nose. The pulse oximeter emits two wavelengths of light to differentiate oxygenated from deoxygenated hemoglobin.
Clinical implications:
SpO2 ≤ 88% indicates the need for supplemental oxygen.
The waveform or pulse rate reading should match the ECG or palpated pulse.
Monitor changes in pulse oximetry during exercise and position changes.
Peripheral vascular disease, arrhythmia (such as atrial fibrillation) sunshine or excessive ambient light, motion artifact, or nail polish may lead to a false reading.
In low-perfusion states, such as hypothermia, hypotension (<80 mm Hg in adults), or vasoconstriction, pulse oximetry may understate oxygen saturation.
Small changes in the percentage of hemoglobin sites chemically combined (saturated) with oxygen (SaO2) can correspond to large changes in the partial pressure of oxygen. Refer to Table 4-6 and Figure 4-7.
Device/Normal Values
Description
Arterial line (A-line)
Normal values:
Systolic: 100-140 mm Hg
Diastolic: 60-90 mm Hg
MAP: 70-105 mm Hg
Purpose:
To directly and continuously record systolic, diastolic, and MAP; to obtain repeated arterial blood samples; or to deliver medications.
Consists of:
A nontapered Teflon catheter. It is placed in the brachial, radial, or femoral artery. The catheter is usually connected to a transducer that converts a physiologic pressure into an electrical signal that is visible on a monitor.
Clinical implications:
If the A-line is displaced, the patient can lose a significant amount of blood at the insertion site. If bleeding occurs from the line, immediately apply direct pressure to the site while calling for assistance.
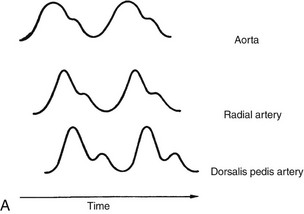
The normal A-line waveform is a biphasic sinusoidal curve with a sharp rise and a gradual decline (Figure 18-4, A). A dampened (flattened) waveform may indicate hypotension, or it may be due to pressure on the line. If a waveform changes during treatment, in the absence of other clinical signs, reposition the patient or limb (if an arterial line is in place) and reassess. If the waveform does not return to baseline, notify the nurse.
A patient with a femoral A-line is usually seen bedside. Hip flexion past 60-80 degrees is avoided. After femoral A-line removal, the patient is usually on strict bed rest for 60-90 minutes, with a sandbag placed over the site.
Upper-extremity insertion sites are usually splinted with an arm board to stabilize the catheter.
The patient with a radial or brachial A-line can usually be mobilized out of bed, although the length of the line limits mobility to a few feet. The transducer may be taped to the patient’s hospital gown at the level of the phlebostatic axis (see Figure 18-3) during mobilization.
Central venous catheter
Normal value:
CVP 2-5 mm Hg or 3-8 cm H2O
Purpose:
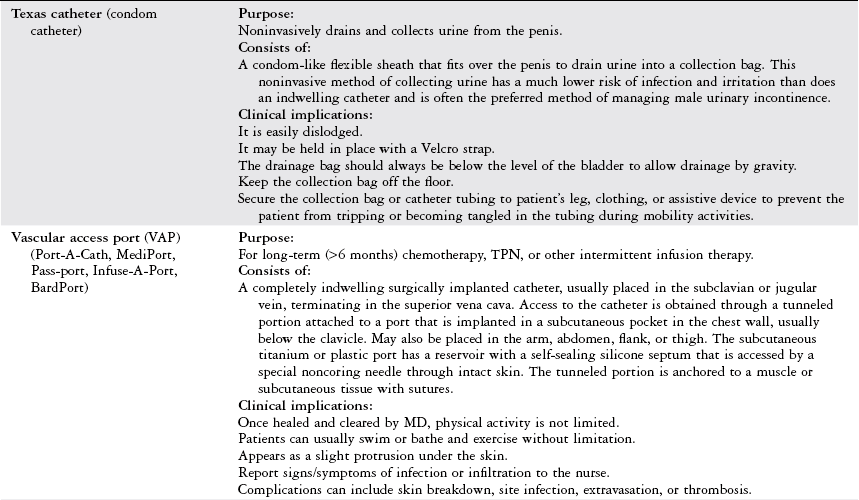
Indicated for a patient with significant fluid volume deficit and is used as a guide to overall fluid balance. The measurement of CVP as a direct reflection of right heart function. (See the Pulmonary artery catheterization section below.) Also provides vascular access for short-term or long-term use (days to months) for parenteral nutrition, repeated blood sampling, administration of vasoactive or caustic drugs or large fluid volumes, or the initiation of transvenous cardiac pacing.
Consists of:
A single-lumen or multiple-lumen intravenous line placed in the subclavian, basilic, jugular, or femoral vein, terminating in the superior vena cava.
Clinical implications:
Do not use a blood pressure cuff on an extremity with a central line. Greatly reduces the need for repeated venipuncture and reduces risk of vein irritation.
A chest x-ray is taken to confirm placement and to rule out iatrogenic pneumothorax.
In the ICU setting, the CVP is connected to a transducer leveled to the phlebostatic line and is measured in mm Hg. In the medical-surgical unit, CVP is measured with a water manometer in cm H2O.
Pacemaker (temporary)
Purpose:
To provide temporary supportive or prophylactic cardiac pacing postoperatively, for bradydysrhythmias (such as heart block), tachydysrhythmias (such as supraventricular tachycardia), for patients post myocardial infarction, for EPS diagnostic studies, or for permanent pacemaker failure. Refer to the Management section in Chapter 3 for more information on pacemakers.
Consists of:
An external pulse generator connected to an insulated electrical lead and 1 to 3 electrodes. The pacer emits an electrical current that directly causes myocardial depolarization. Pacer is set to deliver a certain number of impulses to the heart per minute. There are four basic types.
Epicardial: The wires are placed after heart surgery on the epicardium and exit through a mediastinal incision.
Transvenous: The wires are placed in the right atrium or ventricle via a subclavian or internal jugular central line.
Transcutaneous: Large electrodes are placed (emergently) on the skin over the anterior and posterior chest.
Transthoracic: Wire is placed (emergently) in the right ventricle through a transthoracic needle.
Clinical implications:
The presence of a temporary pacemaker does not, in and of itself, limit functional mobility. However, the underlying indication for the pacemaker may limit the patient’s activity level. Check for mobility restrictions.
Temporary pacing wires and electrodes should be kept dry. Be aware of the location of the generator and wires at all times, especially during mobility activities.
If a temporary pacemaker is placed after a coronary artery bypass graft, the wires are usually removed 1 to 3 days after surgery. The patient is usually placed on bed rest for 1 hour after pacing wire removal, with vital sign monitoring every 15 minutes.
Patients with temporary pacemakers require continuous ECG monitoring to evaluate pacemaker function.
Temporary pacemaker malfunctions can occur and include failure to pace due to a mechanical problem with the pacer, “loss of capture” when the pacing stimulus fails to initiate myocardial depolarization, “rate drift” when pacing occurs at inappropriate times, “undersensing” when the pacer does not detect spontaneous myocardial depolarizations, and “oversensing” when the pacer detects extraneous electrical input, then inappropriately triggers or inhibits output.
Pulmonary artery catheterization (PA line, Swan-Ganz)
Normal values:
PAS: 20-30 mm Hg
PAD: 5-10 mm Hg
PAP (mean): 10-15 mm Hg
PAOP (mean): 5-12 mm Hg
LAP: 5-12 mm Hg
RAP: 2-8 mm Hg
CVP: 2-5 mm Hg
Core temperature: 98.2°-100.2°F (36.8°-37.9°C)
CO: 4-6 lpm (at rest)
CI: 2.2 lpm/m2
Purpose:
To directly or indirectly measure PAS, PAD, PAP, PAOP*, LAP, RAP, CVP, core body temperature, CI, and CO in cases of hemodynamic instability, ARDS, acute myocardial infarction, heart failure, or shock states.
Consists of:
A radiopaque, multilumen balloon-tipped catheter inserted through an introducing sheath into a large vein, usually the subclavian, or the brachial, femoral, or internal jugular vein (see Figure 18-4, B). The catheter is directed by blood flow into various locations of the heart and terminating in the pulmonary artery, with proper placement confirmed by x-ray.
The catheter is connected to a transducer to allow for continuous monitoring.
The proximal lumen opens into the right atrium to measure CVP and CO, and for the delivery of fluids or medications.
The distal lumen opens into the pulmonary artery to measure PAP and to provide access to mixed venous blood samples.
To obtain a PAOP measurement, a balloon at the end of the distal lumen is temporarily inflated. It follows the blood flow from the right ventricle into the pulmonary artery to a distal branch of the pulmonary artery, where it is “wedged” for a short time (up to 15 seconds).
Clinical implications:
The patient with a PA line is usually on bed rest.
Avoid head and neck (for subclavian access) or extremity movements that could disrupt the PA line at the insertion site, including the line dressing.
PAOP is an indirect measure of LAP.
PAP is equal to right ventricle pressure.
RAP is equal to CVP.
CO equals stroke volume (SV) × heart rate (HR).
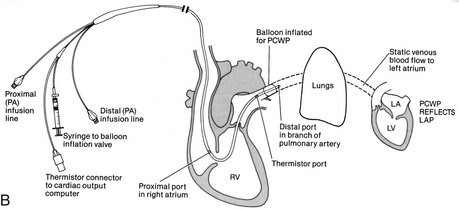
Intracranial Pressure Monitoring
Device
Description
Epidural sensor
Purpose:
To monitor ICP.
Consists of:
A fiberoptic pneumatic flow sensor. It is placed in the epidural space (i.e., superficial to the dura) and connects to a transducer and monitor.
Clinical implications:
The transducer does not need to be adjusted (releveled) with position changes.
Fair to good reliability. Reliability may decrease if the sensor drifts.
A valuable option for patients with severe coagulopathy because it is least invasive.
Subarachnoid bolt
Purpose:
To directly monitor ICP.
Consists of:
A hollow bolt or screw placed in the subarachnoid space through a burr hole.
Clinical implications:
The physician will determine the level at which the transducer should be positioned. This is documented in the chart and posted at the bedside.
The transducer must be repositioned to the appropriate level with position changes.
Poor reliability and decreased accuracy at high ICP readings.
Complications include infection and blockage of the bolt by clot or brain tissue.
Intraventricular catheter (ventriculostomy)
Purpose:
To directly monitor ICP and provide access for the sampling and drainage of cerebrospinal fluid (CSF). Occasionally used to administer medications or to instill air or contrast agent for ventriculography.
Consists of:
A small catheter that is placed in the anterior horn of the lateral ventricle through a burr hole. The catheter connects to a transducer and to a drainage bag, where CSF collects.
Clinical implications:
The nondominant hemisphere is the preferable insertion site.
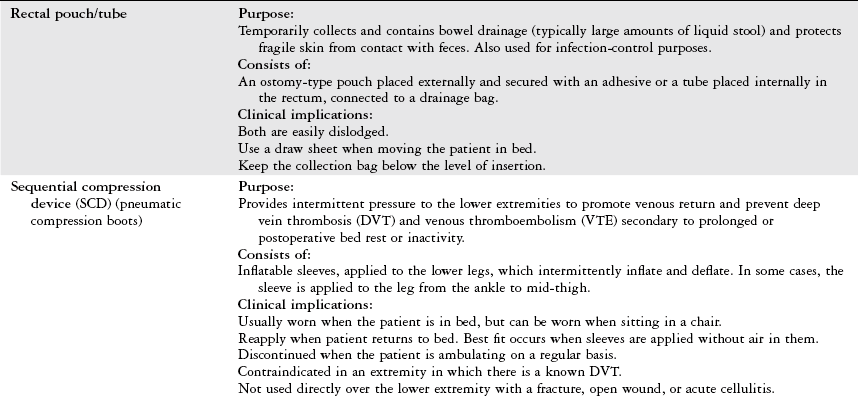
There are two different types of drainage systems: intermittent and continuous.
The intermittent system allows the nurse to drain CSF for 30-120 seconds by momentarily opening a stopcock when the ICP exceeds the parameters set by the physician.
A continuous system allows the drainage of CSF to occur against a pressure gradient when the collection bag is positioned (leveled) above the foramen of Monro. This is usually 15 cm above the external auditory meatus.
The transducer must be repositioned to the appropriate level with position changes.
Very reliable. The gold standard due to a high level of precision.
Complications can include infection, meningitis, ventricular collapse, or catheter occlusion by blood or brain tissue.
Fiberoptic transducer-tipped catheter
Purpose:
To monitor ICP. Can also be used in conjunction with a CSF drainage device (if the catheter is placed in the parenchyma).
Consists of:
A fiberoptic transducer-tipped catheter. It is placed in the ventricle, within the parenchyma, in the subarachnoid or subdural space, or under a bone flap.
Clinical implications:
The transducer does not need to be adjusted (releveled) with position changes.
Very reliable.
Physical Therapy Considerations
Multimodal Neuromonitoring
Technology
Description
Cerebral microdialysis
Purpose:
To measure glucose, lactate, pyruvate, and glycerol levels in the brain of a patient with severe head trauma.
Consists of:
A catheter with a semipermeable distal-end membrane is placed in the brain parenchyma through a burr hole, open craniotomy, or bolt. Ideally placed in the area of injury. Isotonic fluid is pumped in the catheter so that the catheter acts as an artificial blood capillary. Via diffusion, molecules related to the production of ATP are collected and analyzed on an hourly basis or less, depending on patient status.
Clinical implications:
The recovered metabolites represent 70% of the true interstitial fluid concentrations and are compared to normal values.
A result toward a threshold value requires intervention such as permissive hyperglycemia, hypoglycemia prevention, vasopressor use, or positioning.
A second catheter may be placed in an uninjured area of the brain for comparison.
Cerebral blood flow (CBF)
Purpose:
Bedside monitoring of cerebral blood flow and circulation. Perfusion is measured by the ability of brain tissue to carry heat via thermal conduction.
Consists of:
A minimally invasive probe inserted via burr hole is placed in the white matter 2-2.5 cm below the dura in the tissue surrounding the injury. The probe is secured in place with a fixation disc or a bolt. The probe, which has a distal and proximal thermistor and is connected to a cable and monitor, provides a K value that calibrates to body temperature.
Clinical implications:
Reflects real-time perfusion of the brain.
The patient may need to be cooled if brain temperature is >38.5° C.
A second catheter may be placed in an uninjured area of the brain for comparison.
The probe can be seen on computed tomography and radiography, yet is not compatible with magnetic resonance imaging.
Brain tissue oxygenation (PBTO2)
Purpose:
Directly measures brain oxygen and temperature as a marker of cerebral ischemia and secondary brain injury in the mechanically ventilated patient.
Consists of:
A triple-lumen introducer kit with an oxygen sensor at the distal tip (and other sensors for temperature and ICP) is placed 25 to 35 mm into the brain near the injured area via a double- or triple-lumen bolt. A baseline measurement is taken and compared to an oxygen challenge test whereby the patient receives 100% FiO2 for 2-5 minutes. The PBTO2 should increase and is ideally 30 mm Hg.
Clinical implications:
Lower PBTO2 values may represent impending hypoxia.
Elevated PBTO2 values may represent hyperemia or excessive cerebral blood flow, which could increase ICP.
PBTO2 results can be improved during ischemic conditions by decreasing ICP with barbiturates, CSF drainage, or craniotomy.
Cerebral oxygen delivery can be increased with isotonic fluid administration, vasopressors, or blood transfusion. Conditions such as pain, shivering, or agitation that decrease brain oxygenation can be managed with sedatives, antiinflammatory agents, or cooling devices.
Medical-Surgical Management Devices






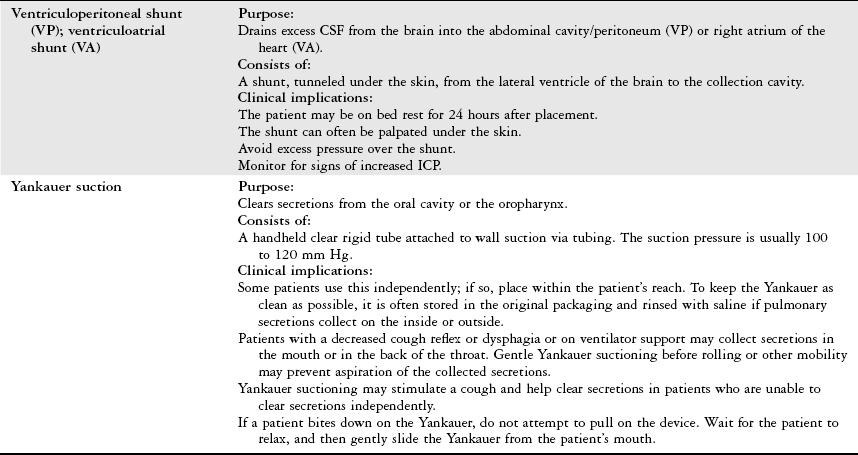
Physical Therapy Considerations
Objectives of Mechanical Ventilation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine




