Malaria
Lawrence M. Barat
Snehal N. Shah
Each year in the United States, more than 7 million people travel to countries where malaria is endemic, approximately 1,400 persons receive diagnoses of malaria, and five to ten persons die from the infection. When malaria is diagnosed rapidly and treated correctly, severe morbidity and mortality from the disease can be prevented. The use of appropriate prevention strategies, including personal protection measures and chemoprophylaxis, can markedly reduce a traveler’s risk of acquiring malaria.
BIOLOGY AND LIFE CYCLE
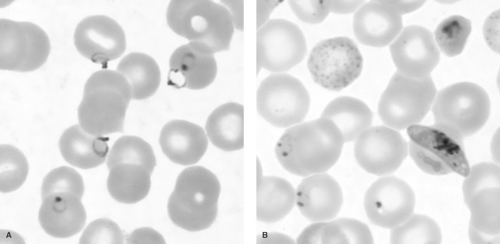
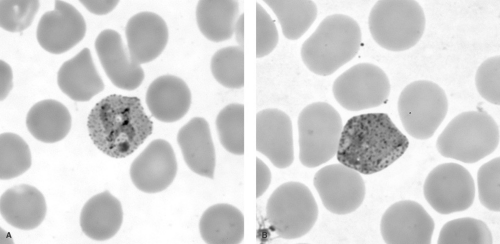
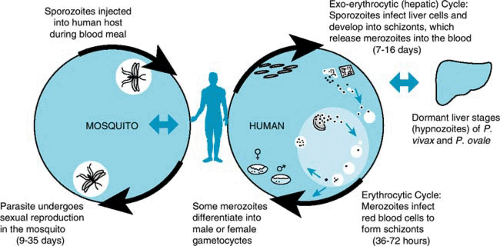
Four species of malaria cause clinical disease in humans: Plasmodium falciparum (Fig. 221.1), P. vivax (Fig. 221.2), P. ovale, and P. malariae. Transmitted by the bite of an infected female Anopheles mosquito during a blood meal, the parasite stage known as the sporozoite enters the blood of the human host and passes rapidly to the liver, infecting hepatocytes (Fig. 221.3). The infecting parasites, now called tissue trophozoites, undergo nuclear division to form schizonts, which yield multiple merozoites. This asexual cycle of the infection is called the exo-erythrocytic cycle and usually lasts from 5.5 to 16.0 days. During this period, the host is asymptomatic. Subsequently, merozoites are released into the blood and rapidly infect erythrocytes.
In the erythrocyte, the parasites undergo a second asexual cycle (erythrocytic cycle); trophozoites develop into schizonts, which produce merozoites. The erythrocytes rupture, releasing merozoites, which then infect other red blood cells. Persons are symptomatic during the erythrocytic cycle; in particular, spiking fevers generally occur with the release of merozoites. After a number of days, the release of merozoites may become synchronized, resulting in classic cyclic fevers. Typically, in P. vivax and P. ovale, merozoites are released in 48-hour cycles (tertian malaria) and, in P. malariae, in 72-hour cycles (quartan malaria).
Some merozoites, after infecting erythrocytes, mature into sexual forms known as gametocytes. Both male and female gametocytes must be ingested during a blood meal by a female Anopheles mosquito for the sexual life cycle to occur in the mosquito’s gut. Sporozoites are produced in the wall of the mosquito’s gut and pass to the salivary glands; they are transmitted when the mosquito takes its next blood meal, thus completing the life cycle.
With P. vivax and P. ovale infections, some liver trophozoites, known as hypnozoites, may remain dormant. Weeks to months, and rarely years, later (the pattern varying by the region in which the infection was acquired), hypnozoites can become active and develop into merozoites. The release of merozoites from the liver results in recurrent parasitemia and clinical illness, termed a relapse. Persons with P. vivax or P. ovale can have multiple relapses for up to 4 years (and occasionally longer) after the primary infection.
Recurrent parasitemia also can occur when parasites persist at low levels in the blood (below the limit of detection)
after the primary attack. The level of parasitemia may increase days to weeks later, causing another clinical attack. These repeat bouts, termed recrudescences, usually occur when patients receive drug treatment that does not eliminate all the blood-stage parasites. Recrudescences can occur with all four species.
after the primary attack. The level of parasitemia may increase days to weeks later, causing another clinical attack. These repeat bouts, termed recrudescences, usually occur when patients receive drug treatment that does not eliminate all the blood-stage parasites. Recrudescences can occur with all four species.
Although the primary mode of transmission is from the bite of an infected Anopheles mosquito, malaria can be acquired from the transfusion of blood or blood products or the transplantation of organs from infected persons. Infection can be acquired congenitally from infected mothers. In the United States, fewer than ten transfusion-associated and congenital infections are diagnosed each year. In both these modes of transmission, persons are infected only with blood-stage parasites. No exo-erythrocytic cycle ensues; therefore, relapses cannot occur.
EPIDEMIOLOGY
The World Health Organization (WHO) estimates that 300 to 500 million clinical cases of malaria are diagnosed worldwide each year, resulting in 1 to 2 million deaths. More than 90% of deaths occur in children younger than age 5 years in sub-Saharan Africa, and malaria is one of Africa’s leading causes of mortality in children under five years of age. P. falciparum is the species responsible for almost all malaria-associated severe morbidity and mortality.
Malaria is transmitted in approximately 100 countries in the tropical and subtropical regions of four continents (Fig. 221.4). The species distribution varies in different regions. In Africa, more than 90% of infections are caused by P. falciparum. In contrast, P. vivax is the predominant infecting species in most
malarious areas in the Americas, Asia, and Oceania. Together, P. falciparum and P. vivax account for more than 90% of all clinical infections worldwide.
malarious areas in the Americas, Asia, and Oceania. Together, P. falciparum and P. vivax account for more than 90% of all clinical infections worldwide.
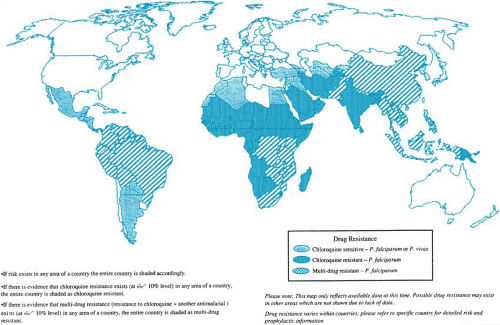 FIGURE 221.4. Worldwide distribution of malaria, including distribution of drug-resistant Plasmodium falciparum, 1998. |
Nearly all of the approximately 1,500 cases of malaria diagnosed yearly in the United States were acquired through international travel and could have been prevented using appropriate chemoprophylaxis. Rarely, malaria is transmitted via blood transfusion, contaminated needles or syringes, or through congenital infection.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
The symptoms and signs of malaria are nonspecific, therefore the clinician must maintain a high index of suspicion and routinely elicit a travel history from patients. Almost all nonimmune persons with malaria will present with fever, either by history or confirmed on physical examination. Malaria should be considered in any patient with fever who has traveled into an area of endemic transmission. Fevers are generally high and spiking, with associated rigors and sweats. Febrile episodes have been described classically as having a predictable periodicity of 48 or 72 hours, with periods of symptomatic improvement between the paroxysms. However, patients with malaria, particularly those with P. falciparum, may not develop cyclic fevers. Therefore, lack of a cyclic fever pattern does not rule out the diagnosis of malaria. Hypotension and tachycardia may accompany the febrile paroxysms, particularly in patients with associated dehydration. Patients with malaria often will describe influenza-like symptoms, including myalgias, malaise, and headache. Diarrhea, vomiting, abdominal pain, and other gastrointestinal symptoms may be present. Cough, shortness of breath, and other respiratory symptoms occur less frequently and may indicate the onset of pulmonary complications or severe anemia. The presence of gastrointestinal or pulmonary symptoms or both should not lead the physician to rule out malaria.
Mild to moderate anemia, resulting in part from destruction of red blood cells or from suppression of erythropoiesis or both, is common and often is accompanied by an elevated reticulocyte count, hyperbilirubinemia, and hemoglobinuria. Inadequate reticulocyte response or microcytic anemia may indicate intercurrent iron deficiency. Mild thrombocytopenia often is present, resulting from peripheral destruction or splenic sequestration, but bleeding diatheses are rare. The leukocyte count can be normal or low. Hepatomegaly and splenomegaly also are common, and liver transaminases may be mildly elevated.
The risk of developing severe and complicated malaria is influenced greatly by whether an infected person has acquired immunity to infection. Persons living in areas that have year-round endemic malaria transmission often develop partial, nonsterilizing immunity after repeated infections. For lifelong residents of these areas, protective immunity typically develops during early childhood or after repeated infections, depending on the intensity of transmission. Persons with acquired immunity still can be infected with malaria parasites, but they generally have a milder febrile illness or may be asymptomatic. As immunity increases, the risk of major complications progressively decreases, although immune persons still may develop
moderate to severe anemia. Immunity to malaria wanes within several months of leaving the endemic area. Persons who live in nonendemic areas, or in areas where transmission is epidemic or seasonal, and persons who have immigrated from malarious areas to nonmalarious areas should be considered nonimmune.
moderate to severe anemia. Immunity to malaria wanes within several months of leaving the endemic area. Persons who live in nonendemic areas, or in areas where transmission is epidemic or seasonal, and persons who have immigrated from malarious areas to nonmalarious areas should be considered nonimmune.
If the disease is left untreated, nonimmune persons with P. falciparum infection are at high risk of developing severe and complicated disease. Distinguishing patients with severe and complicated malaria from those with uncomplicated infection is vitally important, because the risk of death and the treatment strategies used will differ markedly. The risk of complications and mortality from P. falciparum is increased greatly when 5% of red blood cells or more are infected.
Severe disease is defined as the presence of one or more of the following complications in a patient with asexual P. falciparum parasitemia and no other confirmed cause of their symptoms: cerebral malaria, severe anemia, hypoglycemia, renal failure, noncardiogenic pulmonary edema, shock, spontaneous bleeding, hemoglobinuria, or acidemia-acidosis. The manifestations of severe disease most common in young children include cerebral malaria, severe anemia, lactic acidosis, and hypoglycemia. Cerebral malaria can manifest with lethargy progressing to coma or recurrent seizures. Although febrile seizures can occur in young children with malaria, seizures in a patient with P. falciparum infection should be considered evidence of cerebral malaria and require aggressive treatment. Case-fatality rates from cerebral malaria may exceed 40% and, even with optimal treatment, may be as high as 20% to 30%.
Malaria-related severe anemia may be due to multiple causes, including destruction of infected erythrocytes and suppression of erythropoiesis. Moderate to severe hypoglycemia may result from the consumption of glucose by the parasites, hyperinsulinemia, impaired gluconeogenesis, or a combination of these factors. Hypoglycemia usually is responsive to parenteral glucose supplementation. Patients with P. falciparum infection should undergo hemoglobin and blood glucose measurements every 8 to 12 hours during the initial treatment period to monitor for these complications.
Metabolic acidosis is associated with an increased risk of death in severe malaria. The acidosis frequently is attributable to lactic acidosis in children with malaria and severe malarial anemia. Poor tissue perfusion, inadequate oxygen delivery, and dehydration can lead to lactic acidosis, and treatment must be aimed at correcting any reversible cause of acidosis. Noncardiogenic pulmonary edema is an uncommon complication of malaria in children, usually associated with high-density infection. It can manifest as respiratory distress, tachypnea, and nonproductive cough. Chest radiography may demonstrate progressive bilateral interstitial and airspace disease. Respiratory failure can develop rapidly and may progress to death. Acute tubular necrosis is another rare complication of malaria in children, although elevation of blood urea nitrogen and creatinine due to dehydration is common.
Complications with other species are rare. Splenic rupture has been described in patients who have long-standing, untreated P. vivax infection and have developed massive splenomegaly. With effective chemotherapy, this complication is unusual. Nephritis is a rare complication of persistent P. malariae infection, but occurs more commonly in children.
Tropical splenomegaly syndrome, another rare complication in long-term residents of endemic areas, manifests as massive splenomegaly resulting from a hyperimmune response to infection. The diagnosis is supported by the detection of high levels of malaria-specific antibodies in a patient’s serum. Tropical splenomegaly syndrome can be controlled with treatment of the acute infection and continual use of antimalarial chemoprophylaxis while the person remains in the endemic area. Symptoms should resolve, and chemoprophylaxis can be stopped once a person leaves the endemic area.
BOX 221.1. Diagnosis of Malaria
Illustrative Case
Two brothers aged 4 and 8 years were brought to a hospital emergency department. The younger brother was deceased on arrival and the older was in a deep coma. Examination of blood films from both brothers demonstrated P. falciparum infection with more than 10% of erythrocytes parasitized. Three days earlier, the boys had been taken to a local physician because of fevers. The mother told the physician that they had returned recently from a visit to West Africa and had not taken antimalarial chemoprophylaxis. The physician diagnosed a viral infection in both children, and they were sent home without being referred for blood-film examinations.
Conclusion
This case study highlights the importance of considering the possibility of malaria in any person who has fever or influenza-like symptoms and has traveled recently in an area in which malaria transmission occurs. A rapid diagnosis and institution of appropriate antimalarial treatment can prevent the development of the severe morbidity and mortality associated with P. falciparum infection.
This case also emphasizes the importance of obtaining travel histories from all patients with fever. Because the symptoms of malaria can be nonspecific, a history of recent travel in a malarious area may be one of the only clues leading a clinician to consider malaria in the differential diagnosis. Malaria should be considered in any person with fever who has traveled recently in an area in which malaria is endemic.
DIAGNOSIS
For an overview of diagnostic procedures, see Box 221.1.
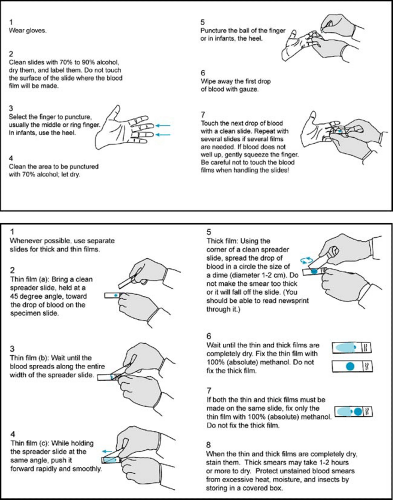
The definitive diagnosis of malaria is based on the identification of parasites infecting erythrocytes on Giemsa- or Wright-Giemsa-stained peripheral blood films. Thick- and thin-blood films should be obtained without delay, because parasites will be present both during and between febrile paroxysms (Fig. 221.5). Thick films allow for the examination of a larger volume of blood, facilitating the detection of low-density infections. Thin films permit easier identification of the infecting species. If parasites are not seen on the initial blood films, repeat samples should be examined 8 hours later. To rule out malaria completely, repeat blood films should be taken every 8 to 24 hours (depending on the severity of a patient’s illness) during the next 72 hours.
If parasites are detected, the examiner must distinguish between P. falciparum and the other species, because falciparum malaria causes almost all malaria-associated severe morbidity and mortality, and the approach to treatment will be different from that of the other three species. Usually, blood films containing P. falciparum will have a predominance of early trophozoites, known as ring forms, with few other developmental stages present. Ring forms derive their name from their signet-ring appearance, with a discrete dot of reddish purple
chromatin and a gray-blue ring of cytoplasm. Later in the course of disease, banana-shaped gametocytes also may be present. Parasite densities greater than 2%, multiple infected erythrocytes, and double-chromatin dot rings are characteristic of P. falciparum, but occasionally can be seen with other species. The presence of mature trophozoites and schizonts suggests infection with other species, although these also can be seen with severe P. falciparum infection. Infected erythrocytes larger than uninfected cells and having a granular cell surface (Schüffner’s granules) would suggest P. vivax or P. ovale. If the infecting species cannot be determined, the patient should be treated presumptively for P. falciparum, because this treatment will be effective against all four species of malaria. Treatment can be changed, if necessary, once the species is confirmed.
chromatin and a gray-blue ring of cytoplasm. Later in the course of disease, banana-shaped gametocytes also may be present. Parasite densities greater than 2%, multiple infected erythrocytes, and double-chromatin dot rings are characteristic of P. falciparum, but occasionally can be seen with other species. The presence of mature trophozoites and schizonts suggests infection with other species, although these also can be seen with severe P. falciparum infection. Infected erythrocytes larger than uninfected cells and having a granular cell surface (Schüffner’s granules) would suggest P. vivax or P. ovale. If the infecting species cannot be determined, the patient should be treated presumptively for P. falciparum, because this treatment will be effective against all four species of malaria. Treatment can be changed, if necessary, once the species is confirmed.
A determination of parasite density should be carried out by counting the number of infected red blood cells and the total number of erythrocytes in ten or more oil-immersion fields in an area in which the erythrocytes form a monolayer on a thin-blood film. Parasite density is calculated by dividing the number of parasitized cells by the total number of erythrocytes. Patients
with P. falciparum infection and a parasite density of 5% or greater are at high risk of complications and death.
with P. falciparum infection and a parasite density of 5% or greater are at high risk of complications and death.
TABLE 221.1. ANTIMALARIAL DRUGS FOR THE TREATMENT OF MALARIA
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|