Leishmaniasis
Murray Wittner
Herbert B. Tanowitz
The leishmaniases are vector-borne diseases caused by obligate intracellular protozoans or hemoflagellates of the genus Leishmania. The diseases affect the viscera, skin, and/or mucous membranes. They are transmitted by several genera and species of phlebotomine sandflies. Three major clinical syndromes are recognized: visceral (VL), cutaneous (CL), and mucocutaneous (MCL) leishmaniasis (Table 220.1). In each type of disease, macrophages are parasitized exclusively.
TABLE 220.1. CLINICAL SYNDROMES CAUSED BY LEISHMANIA SPECIES AND THEIR GEOGRAPHIC DISTRIBUTION* | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The clinical manifestations of leishmaniasis appear to depend upon a complex set of factors, including tropism and virulence of the parasite strain, as well as the susceptibility of the host that may be determined genetically. The host’s cell-mediated immune responses appear to be a major factor moderating these syndromes. Each species of Leishmania has clinical variants that may cause similar patterns of disease in the same host species. Thus, in the case of VL (kala-azar), L. donovani, L. chagasi, L. infantum, and L. amazonensis invade cells of the reticuloendothelial system of the viscera, usually causing splenomegaly, hypergammaglobulinemia, occasionally hepatomegaly, and pancytopenia; untreated, the disease is progressive and usually fatal. In CL, L. tropica (with some notable exceptions), L. major, L. aethiopica, and L. mexicana usually are limited to reticuloendothelial cells of the skin; the infections generally are self-limited, healing spontaneously. Similarly, L. braziliensis invades the reticuloendothelial cells of the skin, although it has the propensity to metastasize to the mucous membranes of the nose, mouth, and pharynx, causing facial disfigurement and, occasionally, death. Strains of L. tropica have been found to cause viscerotropic disease. In individuals with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS), leishmania infections have been recognized as opportunistic infections and may serve as a cofactor in the pathogenesis of HIV infection.
CAUSAL ORGANISM AND LIFE CYCLE (FIG. 220.1)
In vertebrates, leishmania are obligate intracellular organisms that exist only in the amastigote stage. The species that
infect humans usually are morphologically indistinguishable from one another by both light and transmission electron microscopy. The amastigotes are round to oval bodies approximately 2 to 4 μm in diameter; they possess a single nucleus and a specialized mitochondrial structure that has extranuclear DNA, termed a kinetoplast, and they lack a free flagellum. Amastigotes are phagocytized by macrophages and reside within a parasitophorous vacuole of the macrophage. In this environment, they multiply by binary fission, eventually destroying the host cell. The life cycle of the organism is described in Box 220.1. They subsequently are phagocytized, and the process occurs repeatedly. No evidence shows that amastigotes actively penetrate host cells.
infect humans usually are morphologically indistinguishable from one another by both light and transmission electron microscopy. The amastigotes are round to oval bodies approximately 2 to 4 μm in diameter; they possess a single nucleus and a specialized mitochondrial structure that has extranuclear DNA, termed a kinetoplast, and they lack a free flagellum. Amastigotes are phagocytized by macrophages and reside within a parasitophorous vacuole of the macrophage. In this environment, they multiply by binary fission, eventually destroying the host cell. The life cycle of the organism is described in Box 220.1. They subsequently are phagocytized, and the process occurs repeatedly. No evidence shows that amastigotes actively penetrate host cells.
Promastigotes have surface molecules that bind to several macrophage receptors. Within the parasitophorous vacuole, phagocytized promastigotes transform to amastigotes, and multiplication occurs. Many of the invading promastigotes do not survive because mammalian tissue fluids contain cytolytic substances. Those organisms that are phagocytized transform to amastigotes and initiate replication. The organisms can be seen readily in tissues or smears by light microscopy, especially with Giemsa or Wright stain, with which the nucleus
and kinetoplast stain bright red and the cytoplasm stains pale blue. In Novey, McNeal, Nicolle (NNN) culture medium at 24°C, the organisms grow readily, assuming the promastigote or insect form.
and kinetoplast stain bright red and the cytoplasm stains pale blue. In Novey, McNeal, Nicolle (NNN) culture medium at 24°C, the organisms grow readily, assuming the promastigote or insect form.
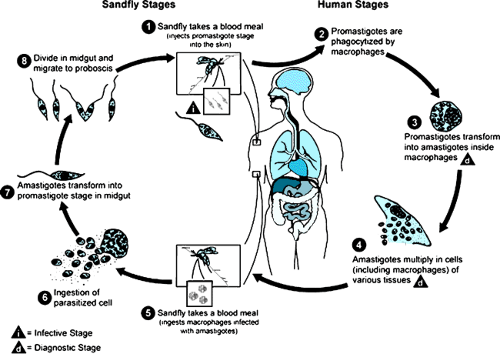 FIGURE 220.1. Promastigote forms from proboscis of crushed sandfly (Phlebotomus). (Courtesy of Dr. Herman Zaiman.) |
Clinical isolates are morphologically indistinguishable, and molecular biologic techniques have been utilized to characterize the strains and species of clinical isolates. These techniques include endonuclease restriction studies of the kinetoplast DNA (K-DNA), buoyant density of the K-DNA and mitochondrial DNA (M-DNA) on cesium chloride, leishmanial isozyme patterns, monoclonal antibody specificity, polymerase chain reaction (PCR) (employing species-specific oligonucleotide primers), and exoantigen secretory 4-factor serotyping.
BOX 220.1. Life Cycle of Leishmania
When a female sandfly feeds on an infected individual, it may ingest an infected cell from blood or tissue. The amastigotes are liberated in the fly’s midgut and, within a few hours, transform to the promastigote stage. These organisms are elongated flagellates, 15 to 25 μm long by 1.5 to 3.5 μm wide, with an anterior, free flagellum that is approximately 15 to 28 μm long and may vary morphologically from short and stumpy to elongated forms. Binary fission occurs within the fly’s midgut, and the large numbers of promastigotes that are produced gradually move forward to the pharynx, buccal cavity, and mouth parts. At 8 to 20 days, depending on temperature and the species of sandfly, the mouth parts of the fly may be blocked partially or completely by large numbers of promastigotes and dislodged into the bite wound when the female sandfly (Phlebotomus spp., Lutzomyia spp.) feeds. In some instances, promastigotes may be inoculated mechanically into the bite wound (Fig. 220.2).
EPIDEMIOLOGY
The precise incidence of leishmaniasis is not known. However, the World Health Organization (WHO) has estimated that 350 million individuals are at risk and that the disease is endemic in more than 88 countries. It occurs in northern Argentine to southern Texas (except Uruguay and Chile) and in southern Europe, Asia (not Southeast Asia), the Middle East, and Africa. The estimated incidence of VL is 500,000 cases annually, and for CL and MCL, it is 1.5 million cases per year. Although the parasite usually is transmitted via the bite of the sandfly, it also may be transmitted as a result of a laboratory accident, direct person-to-person transmission, and blood transfusion. In addition, evidence exists that suggests it may be transmitted either in utero or during the peripartum period.
TYPES OF LEISHMANIASIS
Visceral Leishmaniasis
VL is caused by various organisms in the L. donovani species complex, although recently, strains of L. tropica from the Middle East and L. amazonensis from the New World have been found to cause this syndrome. This disease, which is also known as kala-azar, is found in a broad belt that extends from the Straits of Gibraltar across the Mediterranean through Asia to the east coast of China, at a latitude of between 30 and 48 degrees north. VL has been reported from 47 countries, although Sudan and India account for more than half the cases. In the Western Hemisphere, it is found in Brazil, northern Argentina, Paraguay, Venezuela, Colombia, Guatemala, and Mexico. It is transmitted by various species of sandflies, although occasionally congenital and bloodborne infections also occur.
Kala-azar appears to exist in at least three epidemiologic forms:
A Mediterranean type of VL, with a canine reservoir, in which young children (1 to 4 years of age) are infected, and dogs, foxes, or other feral animals are the reservoirs (L. infantum). This type extends from the Mediterranean littoral through central Asia into China; it also is present in parts of South America (L. chagasi), where foxes and dogs are reservoir hosts. In Brazil, young males most often are infected.
An Indian type (L. donovani) of VL, with a human reservoir, in which the disease predominates in Indian children between 5 and 15 years of age; humans are the only known reservoir. Although sought, evidence of natural infection in dogs has not been found. No evidence of rodent reservoirs exists.
An African type of VL in which rodents are the reservoir hosts. The Nile rat in Sudan and probably the gerbil in Kenya are the reservoirs. In Kenya, kala-azar often is related to old or eroded termite mounds where young males often congregate (Fig. 220.3).
Pathology and Pathobiology
The principal pathologic lesions of VL are caused by reticuloendothelial hyperplasia. A pea-sized granuloma (i.e., leishmaniana) forms at the inoculation site. Subsequently, infected macrophages disseminate to local lymph nodes and then to the viscera, especially the spleen, liver, bone marrow, and intestines. The spleen gradually enlarges, sometimes assuming enormous proportions. Erythrophagocytosis by histiocytes and the anemia so typical of kala-azar may be the result in part of such sequestration of red cells. In early disease, lymphadenopathy caused by large numbers of parasite-filled macrophages is found. Kupffer cells, filled with amastigotes, are swollen and hyperplastic, and centrilobular necrosis or fatty infiltration of the hepatic parenchyma often is observed. In late-stage or chronic disease, increased hepatic fibrosis may give a nodular cirrhotic appearance. The bone marrow may be filled or replaced with parasitized histiocytes, which may cause myelophthisic anemia. Polyclonal B-cell activation results in hyperglobulinemia. This outpouring of humoral antibodies, chiefly IgG and largely nonspecific, is not protective and may represent more than half of the patient’s the total serum proteins.
The outcome of infection appears to depend largely on the host’s capacity to generate a suitable cell-mediated immune response and the virulence of the invading organism (Box 220.2). The genetics of human resistance and susceptibility remains unclear. The role of the sandfly in influencing disease has been appreciated recently. Sandfly saliva contains maxadilan, a potent vasodilatory salivary polypeptide. In Brazil, L. chagasi causes VL, and the sandfly (Lutzomyia longipalpis) that transmits VL has high levels of maxadilan in its saliva, promoting erythema and vasodilation. However, in Costa Rica, L. chagasi causes CL, and the sandfly (L. longipalpis) that transmits L. chagasi has very little maxadilan in its saliva, causing minimal vasodilation and erythema; the organisms multiply in the skin.
Clinical Manifestations
The clinical prepatent period varies widely from 6 weeks to 6 months but has been reported to be as early as 10 to 14 days and as long as 10 years. The primary skin nodule often is not noticed, although it is a more regular feature in African leishmaniasis.
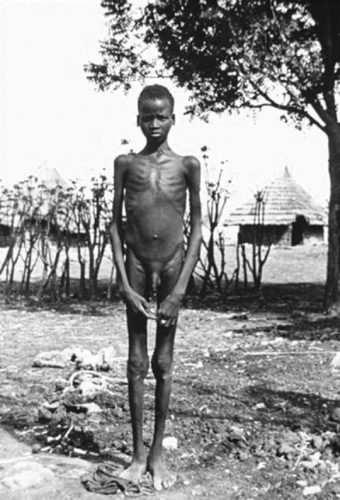
Infantile VL may begin either suddenly with high fevers and vomiting or insidiously with irregular daily fever, anorexia, weight loss, lassitude, and pallor. Double daily fever spikes are a characteristic sign, the fever reaching more than 40°C. Splenomegaly gradually becomes evident by the end of the first month (Fig. 220.4). If the symptoms continue unabated, the spleen may extend to the umbilicus or even into the pelvis. Diarrhea or frank dysentery is not an unusual occurrence, and blood sometimes is observed. A general bleeding diathesis often becomes evident shortly before death occurs. After several months, if the disease goes untreated, patients usually die. Acute fulminant disease is seen more often in infants and young children.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree