Instability Associated with Bone Loss
If you ain’t got a choice, be brave.
Recognizing and properly addressing bone defects is crucial to achieving good surgical outcomes in shoulder instability. One of the most important requirements for glenohumeral stability is a long congruent articular arc in which the humerus and glenoid remain in contact throughout motion. Loss of this congruent arc can occur from glenoid bone loss or defects in the posterior humeral head (i.e., Hill-Sachs lesions) (Figs. 13.1 and 13.2). Such lesions are present in up to 95% of patients with recurrent shoulder instability (1). Examining the glenoid alone, Sugaya et al. (2) reported that 90% of patients with recurrent instability have glenoid bone abnormalities (including bone loss or abnormal contour). Glenoid bone loss was seen in 50% of cases, in over half of which the defect was >5% of the glenoid width. Thus, while many of these bone defect are small, a substantial number of patients will have lesions that are large enough to compromise glenohumeral stability by altering the congruent arc.
In 2000 Burkhart and DeBeer demonstrated a 67% recurrence rate following arthroscopic capsulolabral repair in patients with an inverted-pear glenoid (i.e., loss of 25% of the inferior glenoid diameter) or an engaging Hill-Sachs defect (3). By comparison, individuals without significant bone loss had a 4% recurrence rate with arthroscopic repair. Similar findings were reported by Boileau et al. who noted a 75% rate of recurrence with arthroscopic Bankart repair in the setting of >25% glenoid bone loss (4). In 2007, Burkhart et al. reported on the use of Latarjet in 102 individuals with significant bone loss (5). At a mean follow-up of over 4 years, the recurrence rate was only 4.9%. These studies demonstrate that recognizing significant bone loss and altering the treatment approach accordingly is the most important factor in preventing recurrent instability following surgical stabilization.
EVALUATION FOR BONE LOSS
Our evaluation for bone loss is based on preoperative and intraoperative assessments. We routinely obtain AP, transscapular lateral, and axillary radiographs of the glenohumeral joint. Radiographs are evaluated on all patients for the presence of glenoid bone loss or a Hill-Sachs lesion (Fig. 13.3). While plain radiographs can grossly demonstrate bone defects, the severity is often underestimated. We therefore obtain a computed tomography (CT) scan with three-dimensional (3D) reconstructions on all individuals with suspected bone loss. Additionally, we have a low threshold for obtaining a CT in patients without plain radiographic evidence of bone loss but otherwise having risk factors for recurrence (e.g., young patients, multiple dislocations). To estimate glenoid bone loss, bilateral 3D CTs are obtained. Assuming a normal contralateral shoulder, the percentage of bone loss is easily estimated by comparing the width of the glenoid on the affected side to the width of the glenoid on the normal shoulder in the en face view (Fig. 13.4). We previously reported that in 96% of cases, this technique accurately stratified glenoid bone loss as less than or greater than >25% of glenoid width (6).
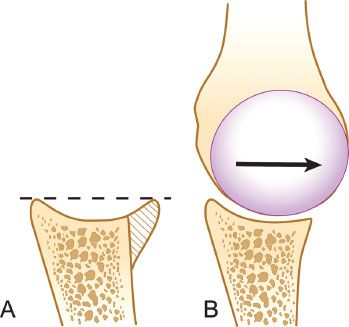
Figure 13.1 A: The anterior glenoid rim serves to “deepen the dish” of the glenoid and acts as a buttress to resist dislocation. B: A glenoid with bone loss has a decreased congruent arc with less resistance to shear forces and less resistance to obliquely applied off-axis loads.
We perform an arthroscopic assessment of bone loss in all patients with instability who are managed surgically. The patient is placed in the lateral decubitus position. Through an anterosuperolateral viewing portal the width of the inferior glenoid is assessed with a calibrated probe inserted through the posterior portal (Fig. 13.5) (7). The bare spot of the glenoid marks the center of the inferior glenoid and is used to compare the posterior glenoid diameter to the anterior diameter. The posterior proximal humerus is assessed for the presence and severity of a Hill- Sachs lesion. A calibrated probe can be used to estimate the depth of the lesion (Fig. 13.6A). The arm is then removed from traction and placed in a position of 90° of abduction and 90 of external rotation to assess for an engaging Hill-Sachs lesion (Fig. 13.6B). If either glenoid bone loss of >25% or an engaging Hill-Sachs lesion in the 90-90 position is noted, we next address any associated pathology that is amenable to arthroscopic repair (e.g., superior labrum anterior and posterior [SLAP] tear), close the wounds, then reposition and redrape in a modified beach-chair position and perform a Latarjet.
One might ask, why perform an arthroscopic evaluation in patients with a 3D CT indicating >25% bone loss? First, although it is highly predictive, the 3D CT is not 100% accurate. Second, utilizing arthroscopy prior to Latarjet, we have shown that 73% of patients have associated intra-articular pathology including a SLAP tear in 64% of cases (8). Given the contribution of the superior labrum to glenoid stability, we feel that it is important to repair a SLAP tear in all cases of glenohumeral instability, and this is best done arthroscopically.
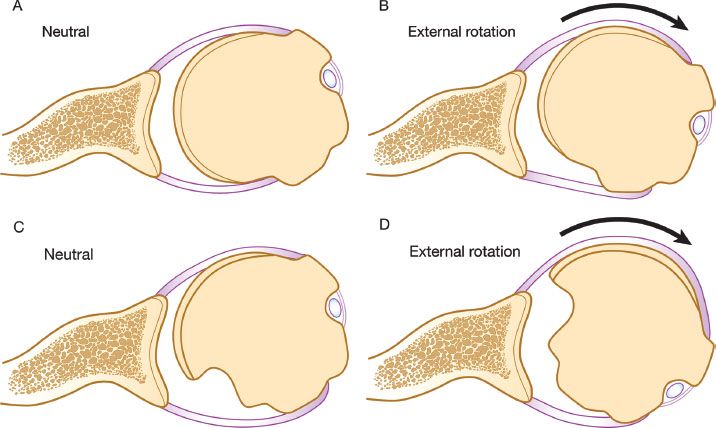
Figure 13.2 A: Normal relationship of the glenoid and humeral articular surfaces. B: Full external rotation still maintains contact between the humeral and glenoid articular surfaces. C: Large Hill-Sachs lesion creates an articular arc length mismatch. D: A small amount of external rotation will cause the Hill-Sachs lesion to engage the anterior corner of the glenoid.
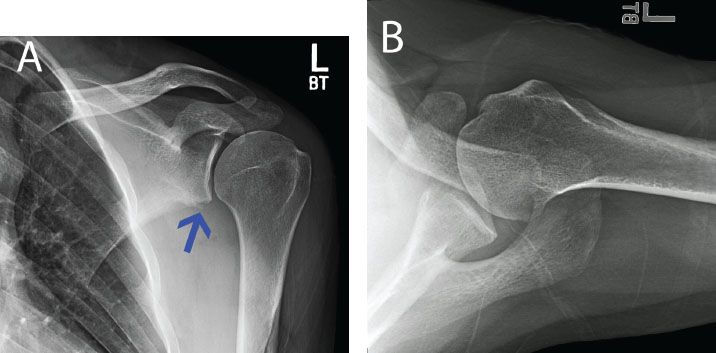
Figure 13.3 A: AP with a radiograph of a left shoulder in a patient with recurrent instability. There is loss of the inferior glenoid cortical rim (blue arrow) suggesting glenoid bone loss. B: An axillary radiograph is also suggestive of anterior glenoid bone loss, but the amount cannot be well quantified.
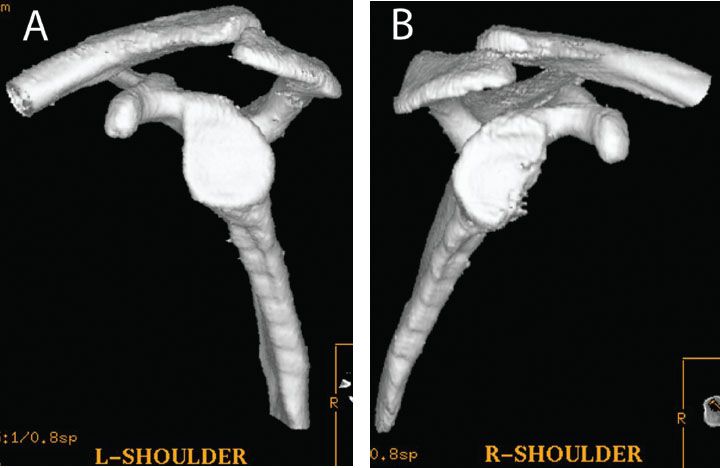
Figure 13.4 Glenoid bone loss can be quantified with a three-dimensional CT of the (A) normal and (B) affected extremities. The percentage of glenoid bone loss can be easily calculated on the en face view by comparing the inferior glenoid diameter of the normal side to that of the affected side.
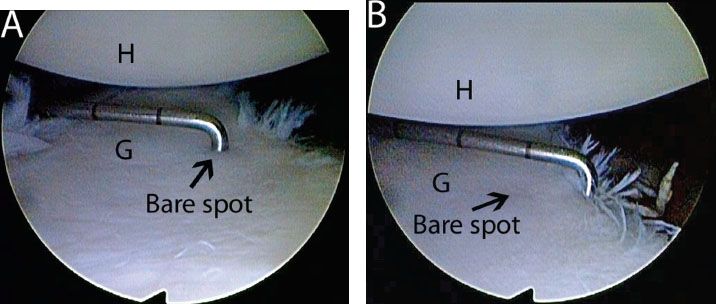
Figure 13.5 Measuring glenoid bone loss arthroscopically from an anterosuperolateral viewing portal in a left shoulder. A: A calibrated probe introduced from a posterior portal marks the distance from the glenoid bare spot to the posterior rim. In this case, the posterior distance is approximately 12 mm. B: The probe is used to measure the distance from the remaining anterior glenoid rim to the glenoid bare spot. In this case, the distance is 6 mm. Thus, there is 6 mm of bone loss anteriorly, or 25% loss of the inferior glenoid diameter. G, glenoid; H, humerus.
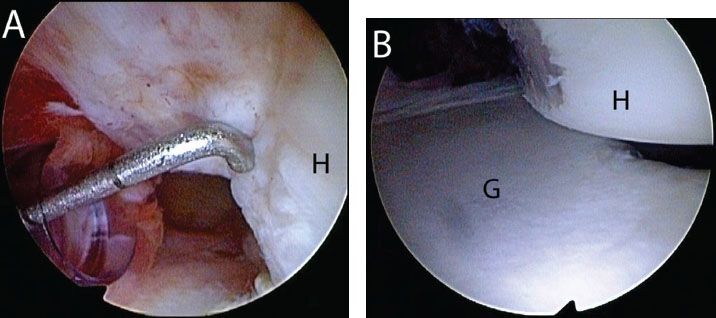
Figure 13.6 Evaluation of a Hill-Sachs lesion from an anterosuperolateral portal in a left shoulder. A: A calibrated probe introduced from a posterior portal is used to estimate the depth of the lesion. In this case, the defect is 5 mm deep. B: The arm is removed from traction and placed in abduction and external rotation to determine whether the lesion engages the anterior glenoid. In this case, the Hill-Sachs lesion engages the glenoid rim. G, glenoid; H, humerus.
Others have asked, why perform the arthroscopy in the lateral decubitus position when the patient has to be repositioned and redraped in the beach-chair position to perform a Latarjet? First, we perform all shoulder arthroscopy in the lateral decubitus position and do not want to compromise our technique. Second, the addition of the arthroscopic remplissage, as discussed below, has increased our armamentarium of surgical options and blurred the lines between which patients require an open Latarjet and which patients can be managed arthroscopically. For example, a patient may have borderline bone loss of 23% on a preoperative CT. If arthroscopy reveals that the bone loss is actually 26%, we would perform a Latarjet. On the other hand, if arthroscopic bone loss is measured to be 20% and the patient is otherwise in a lower category of risk for recurrence, we would perform an arthroscopic Bankart repair and remplissage of the Hill-Sachs, which we strongly believe is best performed in the lateral decubitus position.
LATARJET RECONSTRUCTION
Indications
Since 1996, our indications for performing an open Latarjet procedure have remained the same. They are
or
or
In general, we have found that a large engaging Hill-Sachs lesion usually occurs in combination with an inverted-pear glenoid, so such a case satisfies both indications. Also, when there is a large Hill-Sachs lesion, the coracoid bone graft in the Latarjet procedure will lengthen the articular arc to such an extent that the Hill-Sachs lesion will not be able to engage the glenoid rim. In this way, the Latarjet procedure effectively addresses the Hill-Sachs lesion without the need for an additional bone graft to the humeral defect.
One relative indication for the Latarjet reconstruction is in the patient with severe soft tissue loss involving the anterior labroligamentous complex. Such soft tissue deficiency can occur due to thermal capsular necrosis, or due to multiple failed soft tissue procedures for instability. Although some authors have recommended soft-tissue allografts, we have preferentially done the Latarjet reconstruction without soft tissue augmentation. In this way, we prevent recurrent anterior dislocation by lengthening the glenoid articular arc, as well as providing the “sling effect” of the conjoined tendon, which resists anterior translational forces as the arm goes into abduction and external rotation. The idea for utilizing the Latarjet procedure to address soft tissue loss came from the intraoperative observation that after the coracoid bone graft was secured in place, the shoulder could not be manually dislocated with a significant anteriorly directed force applied by the surgeon, even though the capsule had not yet been repaired. That is, the stability of the Latarjet construct was not at all related to the integrity of the anterior capsule.
Alternatively, we have noted that there are occasional cases with partial loss of the capsule (thermal capsular necrosis; multiple failed surgeries) without any significant bone loss. We have found that such cases may be amenable to arthroscopic repair utilizing a flap of the deep surface of the subscapularis to augment or to substitute for the anterior capsule (see Chapter 12, “Repair of Anterior Instability without Bone Loss”).
Evolution of the Coracoid Bone Graft Technique
Coracoid bone grafting for anterior instability has a long history among French orthopaedic surgeons. We are grateful to our French colleagues, Dr. Gilles Walch and Dr. Johannes Barth, for communicating its history to us, because this story is not readily available in the English-language orthopaedic literature.
Trillat was the first to treat anterior instability with coracoid reinforcement. He did a partial coracoid osteotomy, leaving the inferior cortex intact and then levering the coracoid with an osteotome to create a greenstick fracture through the inferior cortex. A screw then secured the coracoid as an anterior buttress against the subscapularis, tensioning the subscapularis to provide resistance to anterior translation of the humerus.
Latarjet further developed this concept by detaching the pectoralis minor from the coracoid and incising the coracoacromial ligament, leaving a stump of the coracoacromial ligament attached to the coracoid, and then completing the osteotomy at the base of the coracoid so that it could be placed as a bone graft against the anterior glenoid neck. The coracoid was passed through a split in the subscapularis and positioned so that its inferior surface was in contact with the anterior glenoid neck, where it was secured with two screws (Fig. 13.7). In doing so, the posterolateral surface of the coracoid was placed adjacent to the glenoid joint surface.
Patte attributed the success of the Latarjet procedure to a so-called triple effect composed of
Allain et al. (9) looked at the effect of the position of the coracoid graft on long-term results. They found that the best results were in the group of patients in which the lateral edge of the coracoid graft was placed flush with the articular surface of the glenoid. If the coracoid was placed medial to this ideal location, there was an increase in the rate of recurrent dislocation and subluxation; if it was laterally placed, there was a high rate of late degenerative change in the glenohumeral joint.
Burkhart and DeBeer further modified the Latarjet technique, developing the “Congruent-arc Latarjet Procedure,” and they first reported on it in 2000 (3). This technique incorporated two important modifications:
The original congruent-arc technique required freehand positioning of the coracoid graft. We now use an instrumented Latarjet guide system (Arthrex, Inc., Naples, FL) to assure accurate and reproducible positioning of the coracoid graft.
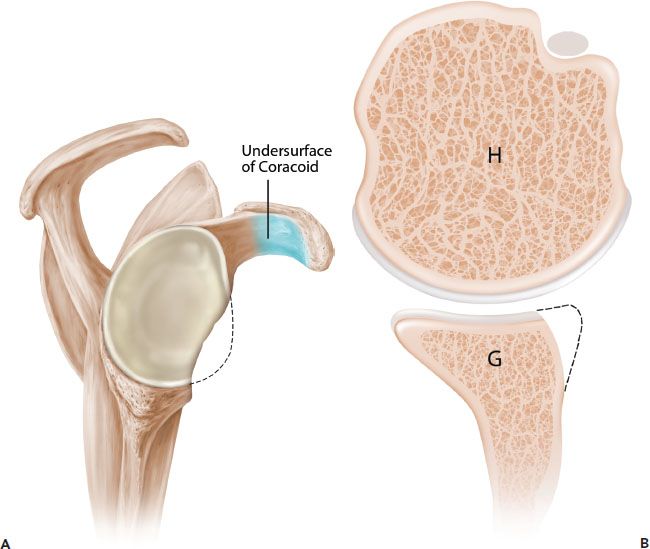
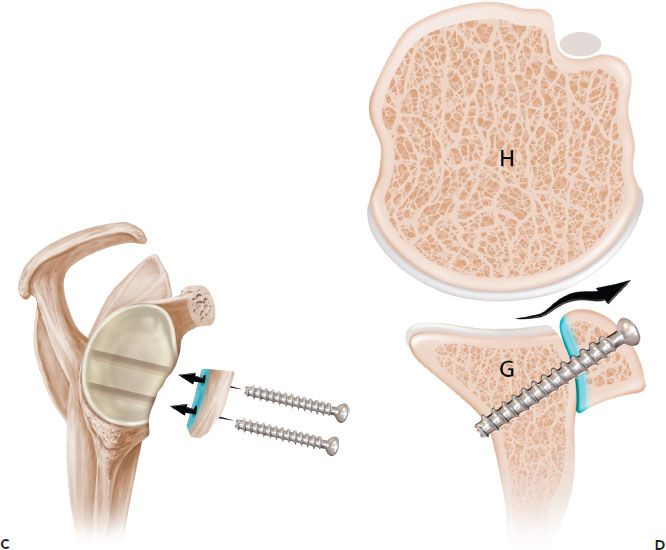
Figure 13.7 Schematic of the French technique for Latarjet reconstruction. Sagittal (A) and axial (B) schematics prior to latarjet reconstruction. C,D: The coracoid is osteotomized and the undersurface of the coracoid is fixed directly to the glenoid. The contour of the coracoid graft does not match the contour of the native glenoid. G, glenoid; H, humerus.
Surgical Technique of Congruent-arc Latarjet Reconstruction
We call our surgical technique the congruent-arc technique because we place the coracoid graft in an orientation such that the arc of its inferior surface is a congruent extension to the glenoid articular arc.
We always perform diagnostic arthroscopy just prior to the Latarjet in order to accurately measure the amount of glenoid bone loss, to assess the Hill-Sachs lesion, and also to evaluate the joint for additional pathology, particularly SLAP lesions. We have found an a 64% incidence of SLAP lesions in our patients who are undergoing Latarjet reconstruction (8). In these cases, we perform an arthroscopic SLAP repair with the patient in the lateral decubitus position. Then, we turn the patient supine and adjust the table to a modified beach-chair position, then re-prep and redrape for the open Latarjet.
Coracoid Osteotomy
In performing the congruent-arc Latarjet, a standard deltopectoral incision is used. The cephalic vein is preserved and retracted laterally with the deltoid muscle. The coracoid is exposed from its tip to the insertion of the coracoclavicular ligaments at the base of the coracoid. The coracoacromial ligament is sharply dissected from the lateral aspect of the coracoid, and the pectoralis minor tendon insertion on the medial side of the coracoid is also sharply dissected from the bone (Fig. 13.9). The medial surface of the coracoid, from which the pectoralis minor is detached, is the surface that will later be in contact with the anterior glenoid neck when the graft is secured by screws.
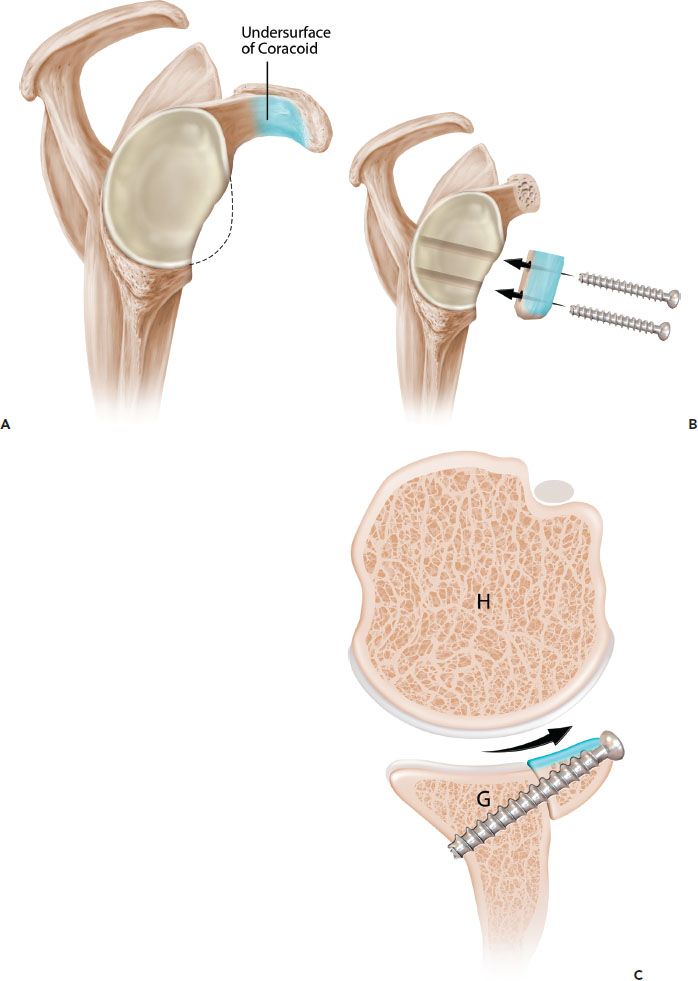
Figure 13.8 Schematic of the Burkhart-DeBeer modification of the Latarjet reconstruction. A: Sagittal view demonstrates glenoid bone loss. The undersurface of the coracoid is shaded in blue. B: Following coracoid osteotomy, the graft is rotated 90° on its long axis, so the undersurface of the coracoid is flush with the glenoid and forms a continuation of the concave glenoid articular arc. The graft is secured with two screws. C: Axial view demonstrates how the orientation changes (compared to the original French technique [Fig. 13.7]) provides a contour that more closely matches the native glenoid concavity and also provides greater length extension of the articular arc. G, glenoid; H, humerus.
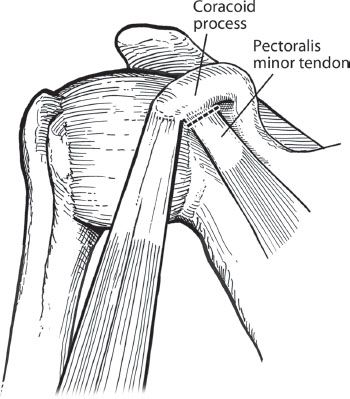
Figure 13.9 In preparation for coracoid osteotomy, the pectoralis minor tendon is sharply dissected off the medial edge of the coracoid.
For the coracoid osteotomy, two options are available. Option 1 involves the use of an osteotome to create the osteotomy (Fig. 13.10A). We believe that an osteotome should be used only in thin patients. In a muscular patient with a large deltoid and pectoralis major, the bulk of these muscles may prevent a proper angle of approach anterior to the glenoid, resulting in the possibility of intra-articular glenoid fracture. Option 2, for muscular patients, involves the use of an angled saw blade to create the osteotomy (Fig. 13.10B). Neurovascular structures are protected by retractors medial and inferior to the saw blade. With either technique, the osteotomy is made just anterior to the coracoclavicular ligaments in order to obtain as much length to the coracoid graft as possible. A graft measuring 2.5 to 3.0 cm in length is ideal, though in small patients a graft of 2.0 cm is adequate for fixation with two screws.
The conjoined tendon is left attached to the coracoid graft to maintain vascularity of the graft and to augment stability of the glenohumeral joint by providing a sling effect upon completion of the procedure. After mobilization of the coracoid and conjoined tendon, the musculocutaneous nerve is protected by retracting the coracoid medially, thereby preventing any stretch injury to the nerve.
Glenohumeral Joint Exposure
Once the coracoid has been osteotomized, there is a clear view of the anterior shoulder. The upper half of the subscapularis tendon is detached distally and reflected medially (Fig. 3.11). The insertion of the lower half of the subscapularis is preserved. After detachment of the upper subscapularis tendon, the plane between lower subscapularis tendon and anterior joint capsule is developed.
Alternatively, the glenoid may be exposed by using a subscapularis split approach. A deep Gelpi retractor is used to spread the split in the muscle. The subscapularis split is made through the muscular fibers at the junction of the superior and middle thirds of the muscle. The capsule is bluntly dissected from the subscapularis, and then the capsular incision is made. We prefer not to use the subscapularis-splitting approach because visualization can be quite limited, and the position of the split severely limits the surgeon’s ability to change the position of the graft on the glenoid if needed.
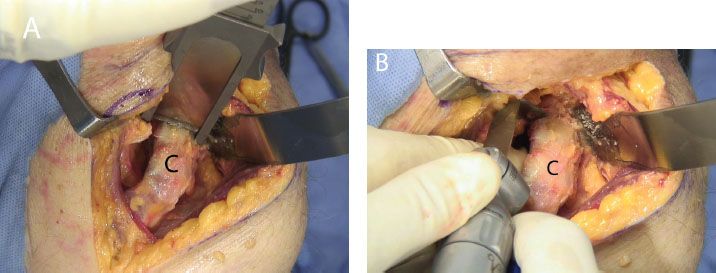
Figure 13.10 Coracoid osteotomy may be performed with (A) an osteotome, or (B) an angled saw blade. C, coracoid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








