Fig. 4.1
Reports to the Danish Medicines Agency of ADRs associated with LA sold in cartridges, 1995–2007. Reports from 2005 may be influenced by peaking sales figures through 2003 and 2004. Decline of reports from 2006 and 2007 probably reflecting change in application pattern (avoidance of articaine for IAN blocks) [6] (Source: Danish Medicines Agency)
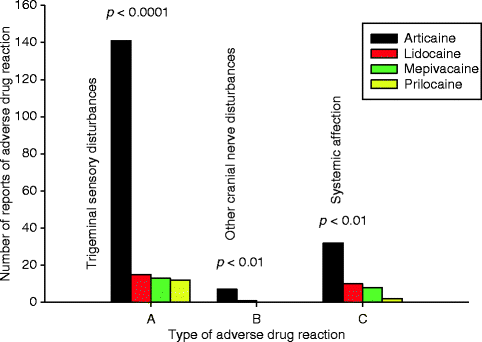
Fig. 4.2
Reports of ADRs associated with LA sold in cartridges in Denmark 2001–2007 distributed based upon type of symptom. Group A shows trigeminal and gustatory sensory disturbances, group B shows other facial nerve affections, and group C illustrates systemic and local non-neurologic ADRs. Significant overrepresentation of articaine was identified in all groups [6] (Source: Danish Medicines Agency)
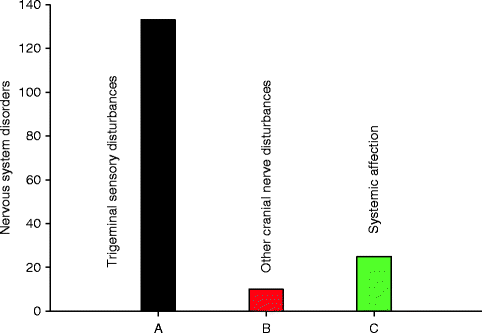
Fig. 4.3
Nervous system disorders associated with articaine, n = 168 (Source: Periodic Safety Update Report, European Medicines Agency 2006–2009 [36])
Comparative data from 2001 to 2007 showed that reports of NSD associated with 4 % articaine-based formulations were significantly more frequent than those of other LAs, and articaine-related NSDs were significantly more numerous than would be expected from the proportion of its market share (Table 4.1). NSDs with lidocaine and prilocaine were equally underrepresented, whereas mepivacaine-related NSDs closely followed the market share. These data show a remarkable similarity with the data of Garisto et al. [12] and Gaffen et al. [11]. Differences in the rate of prilocaine-associated NSD between North America and Europe [12] may be explained by the fact that prilocaine-based formulations in North America are 4 % and the vasoconstrictor is adrenaline, whereas the concentration of prilocaine in Europe is 3 % and the vasoconstrictor is felypressin.
Table 4.1
Reports to the Danish Medicines Agency of trigeminal neurosensory disturbances (NSD) associated with local anesthetics sold in cartridges, 2001–2007 [6]
Local anesthetic | Number of reports (%) | Market shareof drug (%) | P value | Salesvolume (l) | Ratio NSDto liters | Relative risk for articaine | |
|---|---|---|---|---|---|---|---|
Articaine 4 % | 141 (77.9) | 41.2 | <0.001a | 12,660 | 1:90 | Versuseach drug | Versusall drugs |
Mepivacaine 2–3 % | 13 (7.2) | 11.8 | =0.06 | 3,631 | 1:279 | 3.1 | 5.0 |
Prilocaine 3 % | 12 (6.6) | 19.4 | <0.001b | 5,957 | 1:496 | 5.5 | |
Lidocaine 2 % | 15 (8.3) | 27.7 | <0.001b | 8,512 | 1:568 | 6.3 | |
4.3 Clinical Features and Nerve Involvement
A number of studies indicate that the LN is by far the most frequently involved trigeminal branch in LA-associated injuries followed by the IAN [5–7, 9]. This may be explained by the fact that the LN is more superficial in the pterygomandibular space than the IAN, and upon mouth opening, the LN is stretched towards the surface mucosa, making it more vulnerable to injury (mechanic and/or chemical). Upon initial injection of LA, the LN may become anesthetized first, and if multiple needle passes are made, a shock-like sensation may not be experienced if the needle comes into contact with the LN itself. It has also been well documented that females are affected much more often than males [6, 7, 12], and this may be due to a gender difference in the ability to resolve spontaneously following neural trauma.
The specific individual NSD may be temporary, of short or longer duration, or permanent. A limited number of studies describe temporary NSD [1, 8, 11] with an estimated rate of spontaneous recovery in 80–85 % of affected patients. Of the remaining 15–20 % of patients, less than one-third of these patients experience complete neurosensory recovery. Conversely, in our on clinical sample of 131 patients, only 7 % were temporary, while 20 % were potentially permanent, i.e., non-resolving less than 1 year after injury, and 73 % were permanent, i.e., persisting at 1 year after the injection or later [6].
The clinical symptoms may consist of any alteration of cortical perception of trigeminal afferent input, including gustatory perception transferred through the chorda tympani branch of the facial (VII) nerve. Most cases of NSD associated with local anesthetics are characterized as “paresthesia” in the literature [11, 12, 27]. “Numbness” is another popular term that may cover any sensation between mild paresthesia and complete anesthesia. Chronic pain is frequent in patients with LA-associated NSD [3, 4]. In fact, an array of neurologic discomfort may be perceived by the patients, each characterized by a specific neurological term such as hypesthesia, anesthesia, dysesthesia, allodynia, pain, and abnormalities related to gustation, (hypo-, dys-, or ageusia) [3, 6, 22]. Again, since there is a lack of consistency in the literature regarding standardization of terminology to classify the specific ADRs, it is difficult to determine the exact incidence or to determine spontaneous resolution of NSD.
Among our 42 patients with LN injection injuries in nonsurgical cases [3], 18 (43 %) complained of paresthesia, 9 (21 %) had dysesthesia expressed as burning pain, and 3 (7 %) suffered from mechanical allodynia. Only three patients (7 %) had no neuropathic complaint in addition to their functional loss. Thirty-three patients (79 %) had an altered gustatory perception, partial or total loss of gustatory function, or dysgeusia with an unpleasant taste (metallic, electric shock, etc.). Functional neurosensory loss in patients with LN affections was more disturbing than it was in patients with IAN lesions that concurred with clinical records of nerve function (tactile, thermal, and position sensations).
4.4 Etiology: Needle Lesion or Neurotoxicity?
The pathophysiological mechanisms behind these injuries and NSD may be multifactorial, but two different main causes, mechanical lesion and/or neurotoxic reaction, have been the focus of interest [28]. Methodological obstacles have hindered the search for the etiology of LA-associated trigeminal injury, such as randomized controlled trials (RCTs) being not feasible due to the rarity of these injuries, ethical issues, underreporting, marketing issues including commercial interests’ denial, and conflicts of interest. These barriers have precluded a simple explanation of the precise etiology of these injuries. Likewise, lack of proof of the significance of the needle injury, attitudes towards neurotoxicity as a causative mechanism based on emotional or commercial bindings, and denial beyond measurable evidence have confounded the issues, as well [26, 28–31]. Given the assumption of an equal safety profile for all LAs in current use, a distribution of ADRs including NSD would be expected to mirror the market share of each anesthetic, and such a distribution would be suggestive of needle lesion etiology, whereas a distribution disproportionate to market shares would indicate differences in safety profile (neurotoxicity) [6].
Needle Lesion—Theoretically, direct mechanical needle lesion may cause severance of axons, maybe even some of the fascicles within the nerve itself. Such a lesion would expectedly be reflected in a patchy pattern of NSD affecting the area of innervation of each affected neuron/fascicle, not the distribution of the entire nerve branch. Furthermore, mechanical injury to the vasa nervorum may cause hypoxic nerve damage or reactions associated with intraneural hematoma formation, organization with granulation and scar tissue, or toxic blood decay. Local anesthetic-associated NSD in general affects the entire distribution of the affected nerve branch. Pogrel and colleagues [8] wondered how a needle with a diameter of less than ½ mm could produce “such profound damage to the entire nerve.” Direct needle contact with a nerve may be the cause of a painful “electric shock” experience; however, for the reasons mentioned above, this phenomenon may not be commonly experienced by patients. Harn and Durham [1] considered such a “traumatic episode” the main focus of the mechanism of injury. Conversely, Krafft and Hickel [24] in a prospective study observed an electric shock reaction in only 7 % of more than 12,000 patients upon injection of LA; none of these patents affected complained of subsequent NSD. Hillerup and Jensen [3] reported electric shock reactions in 32 % of patients with LA-induced lingual NSD and 33 % related to IAN injury. Interestingly, the severity of NSD did not differ between those patients that experienced an electric shock and those who did not.
Neither studies in humans nor animals [15, 17, 32] support the idea of physical lesion to be a the mechanism of LA-induced NSD; in fact, surgical exploration of four LNs in patients with nonsurgical paresthesia showed no evidence of physical damage to the nerve caused by the needle [7].
Neurotoxicity—Haas and Lennon [9] noted an increase in reported paresthesia rates after the launch of 4 % formulations of articaine and prilocaine in Canada in 1984 and a significant overrepresentation of these drugs in reports of ADRs. Recent studies from North America and Denmark demonstrate that the distribution of NSDs is disproportionate to the market share of commonly used drugs in both national registry data [11, 12] and clinical data [6]. There is a highly significant overrepresentation of NSD associated with 4 % formulations of articaine and prilocaine in accordance with a previous Canadian study [9], with a corresponding underrepresentation of NSDs due to lidocaine.
The concentration issue of neurotoxicity has been confirmed in several experimental studies [13, 14, 16, 17]. Similarly, the physical trauma of needle penetration with the injection of saline solution did not produce a significant reduction of nerve conduction as reflected in measured amplitudes on stimulation in two animal experiments [16, 17]. Additionally, it has also been shown experimentally in the cadaver model that needle penetration of the trigeminal nerve (both the LN and IAN) is likely to result in the needle passing through the interfascicular space, rather than result in direct fascicular damage [8]. It may be concluded that among the etiologic factors in question, direct needle trauma is of minor importance.
Neurotoxicity appears to be the most significant causative factor considering the disproportionate distribution of ADRs including NSD-to-market share, the correlation of neurotoxic reactions with the concentration of the drug, and animal studies showing concentration-dependent neurotoxicity [14, 15, 17, 33]. The vasoconstrictor must also be considered since the ischemic effect may exacerbate the neural injury by impeding blood flow to heal the damaged area.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








