Chapter 13 The objectives of this chapter are to provide a brief understanding of the following: 1 Clinical evaluation of infectious diseases and altered immune disorders, including physical examination and laboratory studies 2 Various infectious disease processes, including etiology, pathogenesis, clinical presentation, and management 3 Commonly encountered altered immune disorders, including etiology, clinical presentation, and management 4 Precautions and guidelines that a physical therapist should implement when treating a patient with an infectious disease process or altered immunity • Health Care–Associated or Nosocomial Infections (Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Candida albicans, and Coagulase-Negative Staphylococci): 6B, 7A • Antibiotic-Resistant Infections: Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococci, Multi-Drug Resistant Acinetobacter baumannii: 6B, 7A • Upper Respiratory Tract Infections (Rhinitis, Sinusitis, Influenza, Pertussis): 6B, 6F, 6G • Lower Respiratory Tract Infections (Tuberculosis, Histoplasmosis, Legionellosis, Severe Acute Respiratory Syndrome [SARS]): 6B, 7C, 7D • Cardiac Infections: Pericarditis, Myocarditis, Left-Sided Endocarditis, Acute Rheumatic Fever, Rheumatic Heart Disease. See Chapter 3: 6B, 6D • Neurological Diseases: Poliomyelitis, Postpoliomyelitis Syndrome, Meningitis, and Encephalitis: 4A, 5C, 5D, 5G, 6E, 5H, 7A • Musculoskeletal Infections: Osteomyelitis and Its Variations: 4G, 4H, 5H • Skin Infections: Cellulitis, groups A and G Streptococcus, and Staphylococcus aureus: 4E, 6H, 7B, 7C, 7D, 7E • Gastrointestinal Infections: Gastroenteritis, Escherichia coli, Shigella, Clostridium difficile, Salmonella, Rotavirus, Norovirus, Adenovirus, and Astrovirus: Please refer to Chapter 8 • Immune System Infections: HIV, Mononucleosis, Cytomegalovirus Infection, and Toxoplasmosis: 4C, 6B • Sepsis: Bacteremia, Septicemia, and Shock Syndrome (or Septic Shock): 5C, 6F, 6H Please refer to Appendix A for a complete list of the preferred practice patterns, as individual patient conditions are highly variable and other practice patterns may be applicable. A patient may be admitted to the hospital setting with an infectious disease process acquired in the community or may develop one as a complication from the hospital environment. The current terminology is to call this type of infection a health care–associated infection (HAI). In 2002, the estimated number of HAIs in U.S. hospitals was 1.7 million, resulting in about 99,000 deaths.1 The major source of HAI is likely the patient’s endogenous flora, but up to 40% of HAIs can be caused by cross infection via the hands of health care workers.2 An infectious disease process generally has a primary site of origin; however, it may result in diffuse systemic effects that may limit the patient’s functional mobility and activity tolerance. Therefore a basic understanding of these infectious disease processes is useful in designing, implementing, and modifying physical therapy treatment programs. The physical therapist may also provide treatment for patients who have disorders resulting from altered immunity. These disorders are mentioned in this chapter because immune system reactions can be similar to those of infectious disease processes (see Appendix 13-A for a discussion of four common disorders of altered immunity: systemic lupus erythematosus, sarcoidosis, amyloidosis, and rheumatoid arthritis). To facilitate the understanding of infectious disease processes, terminology that is commonly used when referring to these processes is presented in Table 13-1.3–6 TABLE 13-1 Terminology Associated with Infectious Disease Processes A person’s immune system is composed of many complex, yet synergistic, components that defend against pathogens (Table 13-2).3 Any defect in this system may lead to the development of active infection. Patients in the acute care setting often present with acquired factors that can create some or most of these defects, which can ultimately affect their immune system (Box 13-1).4 Congenital factors such as lymphocyte deficiency occur rarely. TABLE 13-2 Components of the Immune System *B cells and T cells can also be referred to as B lymphocytes and T lymphocytes, respectively. Data from NS Rote: Immunity. In SE Heuther, KL McCance, editors: Understanding pathophysiology, ed 2, St Louis, 2000, Mosby, pp 125-150; Marieb EN, editor: Human anatomy and physiology, ed 2, Redwood City, CA, 1992, Benjamin Cummings, pp 690-723; Guyton AC, Hall JE: Textbook of medical physiology, ed 9, Philadelphia, 1996, Saunders, pp 445-455. When an infectious disease process is suspected, a thorough patient interview (history) and physical examination are performed to serve as a screening tool for the differential diagnosis and to help determine which laboratory tests are further required to identify a specific pathogen.7 Monitoring the patient’s temperature over time (both throughout the day and daily) provides information regarding the progression (a rise in temperature) or a regression (a fall in temperature) of the infectious process. With an infectious process, some of the bacteria and extracts from normal leukocytes are pyrogenic, causing the thermostat in the hypothalamus to rise, resulting in an elevated body temperature.8 A fall in body temperature from a relatively elevated temperature may also signify a response to a medication. Most of the evaluation process for diagnosing an infectious disease is based on laboratory studies. These studies are performed to (1) isolate the microorganisms from various body fluids or sites; (2) directly examine specimens by microscopic, immunologic, or genetic techniques; or (3) assess specific antibody responses to the pathogen.9 This diagnostic process is essential to prescribing the most specific medical regimen possible for the patient. During hematologic studies, a sample of blood is taken and analyzed to assist in determining the presence of an infectious process or organism. Hematologic procedures used to diagnose infection include leukocyte count, differential white blood cell (WBC) count, and antibody measurement.10 Leukocyte, or WBC, count is measured to determine whether an infectious process is present and should range between 5000 and 10,000 cells/mm3.3 An increase in the number of WBCs, termed leukocytosis, is required for phagocytosis (cellular destruction of microorganisms) and can indicate the presence of an acute infectious process.11 Leukocytosis can also be present with inflammation and may occur after a surgery with postoperative inflammation.8 A decreased WBC count from baseline, termed leukopenia, can indicate altered immunity or the presence of an infection that exhausts supplies of certain WBCs.11 A decreased WBC count relative to a previously high count (i.e., becoming more within normal limits) may indicate the resolution of an infectious process.11 Five types of WBCs exist: lymphocytes, monocytes, neutrophils, basophils, and eosinophils. Specific types of infectious processes can trigger alterations in the values of one or more of these cells. Detection of these changes can assist in identification of the type of infection present. For example, an infection caused by bacteria can result in a higher percentage of neutrophils, which have a normal range of 2.0 to 7.5 × 109/liter. In contrast, a parasitic infection will result in increased eosinophils, which have a normal count of 0.0 to 0.45 × 109/liter.11 Staining allows for morphologic examination of organisms under a microscope. Two types of staining techniques are available: simple staining and the more advanced differential staining. Many types of each technique exist, but the differential Gram’s stain is the most common.12 Gram’s stain is used to differentiate similar organisms by categorizing them as gram-positive or gram-negative. This separation assists in determining subsequent measures to be taken for eventual identification of the organism. A specimen is placed on a microscope slide, and a series of steps are performed.13 A red specimen at completion indicates a gram-negative organism, whereas a violet specimen indicates a gram-positive organism.13 The purpose of a culture is to identify and produce isolated colonies of organisms found within a collected specimen. Cells of the organism are isolated and mixed with specific media that provide the proper nourishment and environment (e.g., pH level, oxygen content) needed for the organism to reproduce into colonies. Once this has taken place, the resultant infectious agent is observed for size, shape, elevation, texture, marginal appearance, and color to assist with identification.13 When an organism has been isolated from a specimen, its sensitivity (susceptibility) to antimicrobial agents or antibiotics is tested. An infectious agent is sensitive to an antibiotic when the organism’s growth is inhibited under safe dose concentrations. Conversely, an agent is resistant to an antibiotic when its growth is not inhibited by safe dose concentrations. Because of a number of factors, such as mutations, an organism’s sensitivity, resistance, or both to antibiotics are constantly changing.14 Cytology is a complex method of studying cellular structures, functions, origins, and formations. Cytology assists in differentiating between an infectious process and a malignancy and in determining the type and severity of a present infectious process by examining cellular characteristics.12,15 It is beyond the scope of this book, however, to describe all of the processes involved in studying cellular structure dysfunction. A pleural tap, or thoracentesis, is the process by which a needle is inserted through the chest wall into the pleural cavity to collect pleural fluid for examination of possible malignancy, infection, inflammation, or any combination of these. A thoracentesis may also be performed to drain excessive pleural fluid in large pleural effusions.16 Pericardiocentesis is a procedure that involves accessing the pericardial space around the heart with a needle or cannula to aspirate fluid for drainage, analysis, or both. It is primarily used to assist in diagnosing infections, inflammation, and malignancies and to relieve effusions built up by these disorders.17 Synovial fluid analysis, or arthrocentesis, involves aspirating synovial fluid from a joint capsule. The fluid is then analyzed and used to assist in diagnosing infections, rheumatic diseases, and osteoarthritis, all of which can produce increased fluid production within the joint.18 A gastric lavage is the suctioning of gastric contents through a nasogastric tube to examine the contents for the presence of sputum in patients suspected of having tuberculosis. The assumption is that patients swallow sputum while they sleep. If sputum is found in the gastric contents, the appropriate sputum analysis should be performed to help confirm the diagnosis of tuberculosis.16,19 Historically, gastric lavage has also been administered as a medical intervention to prevent absorption of ingested toxins in the acutely poisoned patient, although its use for this purpose is now rarely recommended.20 Peritoneal fluid analysis, or paracentesis, is the aspiration of peritoneal fluid with a needle. It is performed to (1) drain excess fluid, or ascites, from the peritoneal cavity, which can be caused by infectious diseases, such as tuberculosis; (2) assist in the diagnosis of hepatic or systemic malfunctions, diseases, infection such as spontaneous bacterial peritonitis (SBP), or malignancies; and (3) help detect the presence of abdominal trauma.16,19,21 Imaging with plain x-rays, computed tomography scans, positron emission tomography, and magnetic resonance imaging scans can also help identify areas with infectious lesions.22,23 Minuscule amounts of pathogens can be detected by using the molecular biology techniques of enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), and polymerase chain reaction (PCR).24,25 In addition, the following diagnostic studies can also be performed to help with the differential diagnosis of the infectious process. For a description of these studies, refer to the sections and chapters indicated below: Nosocomial infection is an older general term that refers to an infection that is acquired in the hospital setting. Since 2008 the Centers for Disease Control and Prevention (CDC) has used the generic term health care–associated infections instead of nosocomial.6 Many pathogens can cause an HAI, but the most commonly reported bacteria in past years have been Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Candida albicans, and coagulase-negative staphyloccoci.26,27 Patients who are at risk for developing HAIs are those who present with28: 1. Age: the very young or the very old 2. Immunodeficiency: chronic diseases (cancer, chronic renal disease, chronic obstructive pulmonary disease, diabetes, or acquired immunodeficiency syndrome [AIDS]) 3. Immunosuppression: chemotherapy, radiation therapy, or corticosteroids 4. Misuse of antibiotics: overprescription of antibiotics or use of broad-spectrum antibiotics, leading to the elimination of a patient’s normal flora, which allows for the colonization of pathogens and development of drug-resistant organisms 5. Use of invasive diagnostic and therapeutic procedures: indwelling urinary catheters, monitoring devices, intravenous (IV) catheters, and mechanical ventilation with intubation 6. Agitation: Resulting in removal of medical equipment such as central venous catheters or self-extubation of artificial airways 7. Surgery: incisions provide access to pathogens 8. Burns: disrupt the first line of defense 9. Length of hospitalization: increases the exposure to pathogens and medical intervention The mode of transmission for pathogens that cause HAIs can vary from contact to airborne. Pathogens can also become opportunistic in patients who are immunocompromised or immunosuppressed. Common sites for HAIs are in the urinary tract, surgical wounds, joints, and the lower respiratory tract (e.g., pneumonia). Clinical manifestations and management of HAIs vary according to the type of pathogen and the organ system involved. However, the primary management strategy for HAIs is prevention by following the standard and specific precautions outlined in Table 13-3.9,26,29,30 TABLE 13-3 Summary of Precautions to Prevent Infection *These precautions are in addition to practicing Standard Precautions. Data from Rice D, Eckstein EC: Inflammation and infection. In Phipps WJ, Sands JK, Marek JF, editors: Medical-surgical nursing, concepts and clinical practice, ed 6, St Louis, 1999, Mosby, pp 237-245; Anderson KN, editor: Mosby’s medical, nursing, and allied health dictionary, ed 5, St Louis, 1998, Mosby, p 2BA5. The number of antibiotic-resistance infections is growing in health care facilities. Approximately 50% of antibiotic use in hospitals is unnecessary or inappropriate. In response to this problem, the CDC has launched a program called “Get Smart for Healthcare” whose goals include reducing unnecessary antibiotic use (resulting in less antimicrobial resistance), decreasing health care costs, and improving patient outcomes in hospitals and long-term care facilities.31 Microbial experts from the European Centre for Disease Prevention and Control and in the United States from the CDC have recently developed interim standard terminology to describe this resistance.32 They developed three major definitions for resistance: multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) bacteria. The agreed-on definitions are MDR as acquired nonsusceptibility to at least one agent in three or more antimicrobial categories, XDR as nonsusceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e., remaining susceptible to only one or two categories), and PDR as nonsusceptibility to all agents in all antimicrobial categories. Methicillin-resistant S. aureus (MRSA) is a strain of Staphylococcus that is resistant to methicillin or similar agents, such as oxacillin and nafcillin. Methicillin is a synthetic form of penicillin and was developed because S. aureus developed resistance to penicillin, which was originally the treatment choice for S. aureus infection. However, since the early 1980s, this particular strain of S. aureus has become increasingly resistant to methicillin. The contributing factor that is suggested to have a primary role in the increased incidence of this HAI is the indiscriminate use of antibiotic therapy.30,33 In addition, patients who are at risk for developing MRSA infection in the hospital are patients who33–35: • Are debilitated, elderly, or both • Are hospitalized for prolonged time periods • Have multiple surgical or invasive procedures, an indwelling cannula, or both • Are taking multiple antibiotics, antimicrobial treatments, or both MRSA is generally transmitted by person-to-person contact or person-to-object-to-person contact. MRSA can survive for prolonged periods of time on inanimate objects, such as telephones, bed rails, and tray tables, unless such objects are properly sanitized. Hospital personnel can be primary carriers of MRSA, as the bacterium can be colonized in healthy adults. MRSA infections can be diagnosed via nasal swabs.36 Management of MRSA is difficult and may consist of combining local and systemic antibiotics, increasing antibiotic dosages, and applying whole-body antiseptic solutions. In recent years, vancomycin has become the treatment of choice for MRSA; however, evidence has shown that patients with this strain of S. aureus are also developing resistance to vancomycin (vancomycin intermediate S. aureus—VISA).30 Therefore prevention of MRSA infection is the primary treatment strategy and includes the following26,33–35: Vancomycin-resistant enterococci (VRE) infection is another HAI that has become resistant to vancomycin, aminoglycosides, and ampicillin. The infection can develop as endogenous enterococci (normally found in the gastrointestinal or the female reproductive tract) become opportunistic in patient populations similar to those mentioned earlier with MRSA. VRE infections can be diagnosed via rectal swab.26,30,37,38 Management of VRE infection is difficult, as the enterococcus can withstand harsh environments and easily survive on the hands of health care workers and on hospital objects. Treatment options are very limited for patients with VRE, and the best intervention plan is to prevent the spread of the infectious process.30 Strategies for preventing VRE infections include the following37: • The controlled use of vancomycin • Timely communication between the microbiology laboratory and appropriate personnel to initiate contact precautions as soon as VRE is detected • Implementation of screening procedures to detect VRE infection in hospitals where VRE has not yet been detected (i.e., randomly culturing potentially infected items or patients) • Preventing the transmission of VRE by placing patients in isolation or grouping patients with VRE together, wearing gown and gloves (which need to be removed inside the patient’s room), and washing hands immediately after working with an infected patient • Designating commonly used items, such as stethoscopes and rectal thermometers, to be used only with VRE patients • Disinfecting any item that has been in contact with VRE patients with the hospital’s approved cleaning agent Over the past decade Acinetobacter baumannii (AB) has become one of the most difficult pathogens to effectively treat because it easily acquires a wide spectrum of antimicrobial resistance, resulting in the commonly found MDR and the much more serious but fortunately rarer PDR forms. It is a gram-negative coccobacillus that has become one of the most important pathogens, particularly in the intensive care unit (ICU). AB infections in the hospital can cause serious complications such as ventilator-associated pneumonia (VAP), bloodstream infection, wound infections, and nosocomial meningitis.39,40 AB is remarkable in that it is ubiquitous, exists in diverse habitats (e.g., human skin), can survive for long periods of time on dry inanimate surfaces (e.g., hospital bed rails) and as already mentioned can acquire antimicrobial resistance extremely rapidly. These factors combined, especially the latter two, greatly facilitate MDR-AB outbreaks in the ICU, in physical therapy wound clinics and even multi-facility outbreaks.41,42 Fortunately, strict infection-control measures (e.g., contact isolation precautions outlined in Table 13-3 and in guidelines for physical therapy intervention at the end of the chapter) can decrease health care staff and environmental colonization and/or contamination.43 MDR-AB and PDR-AB infections can also be prevented by following the previously mentioned guidelines effective against MRSA and VRE. Infections of the respiratory tract can be categorized as upper or lower respiratory tract infections. Upper respiratory tract infections that are discussed in this section consist of allergic and viral rhinitis, sinusitis, influenza, and pertussis. Lower respiratory tract infections that are discussed in this section consist of tuberculosis, histoplasmosis, legionellosis, and severe acute respiratory syndrome. Pneumonia is the most common lower respiratory tract infection and is discussed under Health Conditions in Chapter 4. Management of allergic rhinitis includes antihistamines, decongestants, nasal corticosteroid sprays, and allergen avoidance. Management of viral rhinitis includes rest, fluids, antipyretics, and analgesics.44–46 Management of sinusitis includes antibiotics (as appropriate), decongestants or expectorants, and nasal corticosteroids.45 Clinical manifestations of influenza include (1) a severe cough, (2) abrupt onset of fever and chills, (3) headache, (4) backache, (5) myalgia, (6) prostration (exhaustion), (7) coryza (nasal inflammation with profuse discharge), and (8) mild sore throat. Gastrointestinal signs and symptoms of nausea, vomiting, abdominal pain, and diarrhea can also present in certain cases. The disease is usually self-limiting in uncomplicated cases, with symptoms resolving in 7 to 10 days. A complication of influenza infection is pneumonia, especially in the elderly and chronically diseased individuals.3,4,16,45 If management of influenza is necessary, it may include the following3,4,16,45: Pertussis, or whooping cough, is an acute bacterial infection of the mucous membranes of the tracheobronchial tree, and recently the number of cases has been increasing in the United States.47 It occurs most commonly in children younger than 1 year and in children and adults of lower socioeconomic populations. The defining characteristics are violent cough spasms that end with an inspiratory “whoop,” followed by the expulsion of clear tenacious secretions. Symptoms may last 1 to 2 months. Pertussis is transmitted through airborne particles and is highly contagious.48 Management of pertussis may include any of the following16,48: • Antiinfective and antiinflammatory medications • Bronchopulmonary hygiene with endotracheal suctioning, as needed • Supplemental oxygen, assisted ventilation, or both • Fluid and electrolyte replacement • Active immunization by vaccines • Respiratory isolation for 3 weeks after the onset of coughing spasms or 7 days after antimicrobial therapy Tuberculosis (TB) is a chronic pulmonary and extrapulmonary infectious disease caused by the tubercle bacillus. It is transmitted through airborne Mycobacterium tuberculosis particles, which are expelled into the air when an individual with pulmonary or laryngeal TB coughs or sneezes.49 When M. tuberculosis reaches the alveolar surface of a new host, it is attacked by macrophages, and one of two outcomes can result: Macrophages kill the particles, terminating the infectious process, or the particles multiply within the WBCs, eventually causing them to burst. This cycle is then repeated for a variable time frame between 2 and 12 weeks, after which time the individual is considered to be infected with TB and will test positive on tuberculin skin tests, such as the Mantoux test, which uses tuberculin-purified protein derivative,* or the multiple puncture test, which uses tuberculin. At this point, the infection enters a latent period (most common) or develops into active TB.49,50 A six-category classification system has been devised by the American Thoracic Society and the Centers for Disease Control and Prevention (CDC) to describe the TB status of an individual.49,51 1. No TB exposure, not infected 2. TB exposure, no evidence of infection 3. Latent TB infection, no disease
Infectious Diseases
Preferred Practice Patterns
Definition of Terms
Term
Definition
Antibody
A highly specific protein that is manufactured in response to antigens and defends against subsequent infection.
Antigen (immunogen)
An agent that is capable of producing antibodies when introduced into the body of a susceptible person.
Carrier
A person who harbors an infectious agent that can cause a specific disease but who demonstrates no evidence of the disease.
Colonization
The process of a group of organisms living together; the host can carry the microorganism without being symptomatic (no signs of infection).
Communicable
The ability of an infective organism to be transmitted from person to person, either directly or indirectly.
Disseminated host
Distributed over a considerable area.
The person whom the infectious agent invades and from whom it gathers its nourishment.
Health care–associated infection (HAI)
Localized or systemic condition resulting from an adverse reaction to the presence of an infectious agents(s) or its toxin(s); there must be no evidence that the infection was present or incubating at the time of admission to the acute care setting.
Immunocompromised
An immune system that is incapable of a normal response to pathogenic organisms and tissue damage.
Immunodeficiency
Decreased or compromised ability to respond to antigenic stimuli by appropriate cellular immunity reaction.
Immunosuppression
The prevention or diminution of the immune response, as by drugs or radiation.
Nosocomial infection
Infection acquired in the hospital setting; note that this has been replaced by HAI (see above).
Opportunistic
An infectious process that develops in immunosuppressed individuals. (Opportunistic infections normally do not develop in individuals with intact immune systems.)
Pathogen
An organism capable of producing a disease.
Subclinical infection
A disease or condition that does not produce clinical symptoms, or the time period before the appearance of disease-specific symptoms.
Body Structure and Function
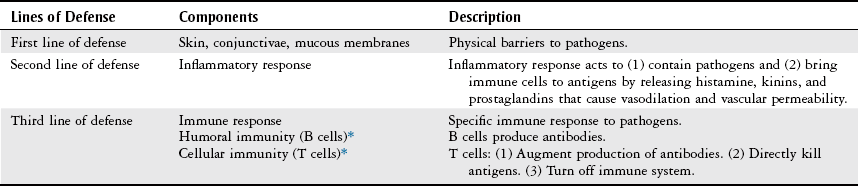
Evaluation
Physical Examination
Vital Signs
Temperature.
Laboratory Studies
Hematology
Leukocyte Count.
Differential White Blood Cell Count.
Microbiology
Staining.
Culture.
Sensitivity and Resistance.
Cytology
Body Fluid Examination
Pleural Tap.
Pericardiocentesis.
Synovial Fluid Analysis.
Gastric Lavage.
Peritoneal Fluid Analysis.
Other Studies
Health Conditions
Health Care–Associated or Nosocomial Infections
Precaution
Description
Standard
Treat all patient situations as potentially infectious. Wash hands before and after each patient contact. Wear a different set of gloves with each patient. If splashing of body fluids is likely, wear a mask or face shield, or both, and a gown.
Airborne*
A mask is required in situations where contagious pathogens can be transmitted by airborne droplet nuclei, as in the case of measles, varicella (chickenpox), or tuberculosis.
Droplet*
A mask or face shield, or both, are required when large-particle droplet transmission (usually 3 ft or less) is likely.
Droplet transmission involves contact of the conjunctivae or the mucous membranes of the nose or mouth with large-particle droplets (larger than 5 µm in size) generated from coughing, sneezing, talking, and certain procedures, such as suctioning and bronchoscopy.
Examples of pathogens requiring droplet precautions are Haemophilus influenzae, Neisseria meningitidis, mycoplasmal pneumonia, streptococcal pneumonia, mumps, and rubella.
Contact*
Gown and gloves are required when pathogens are transmitted by direct person-to-person contact or person-to-object contact. Examples of these pathogens include Acinetobacter baumannii, Clostridium difficile, Escherichia coli, herpes simplex virus, herpes zoster, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus.
Antibiotic-Resistant Infections
Methicillin-Resistant Staphylococcus aureus Infection.
Vancomycin-Resistant Enterococci Infection.
Multidrug-Resistant Acinetobacter baumannii.
Respiratory Tract Infections
Upper Respiratory Tract Infections
Rhinitis.
Sinusitis.
Influenza.
Pertussis.
Lower Respiratory Tract Infections
Tuberculosis.
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Infectious Diseases
Only gold members can continue reading. Log In or Register to continue





