Chapter 19 Immune Function Assessment
 Assessment of the Immune System
Assessment of the Immune System
Background
A variety of tests can be used to evaluate the immune system. To determine what type of immune assessment to use for each clinical diagnosis, it is important to understand how underlying immune physiology contributes to a clinical picture. Clinicians can assess qualitative or functional activity of immune cells. Qualitative data may include things like types, number, and activation states of cells. Cells of the immune system express different proteins on their surface. These proteins, called biomarkers, allow us to identify different cells as well as their activation state. To assess immunodeficiency and lymphoproliferative diseases, the number of T cells and B cells must be quantified. Functional activity of immune cells involves putting the cells into an in vitro assay system, adding antigen or mitogen, and measuring a function such as cytotoxicity, cytokine production, or antibody secretion. Levels of proteins that are produced by lymphocytes and monocytes, such as cytokines and antibodies, can be analyzed as well. Tests of genetic predisposition to disease can be evaluated with genetic tests. An overview of the assays used to measure cells is found in Table 19-1. A description of these tests is found in Box 19-1.
TABLE 19-1 Overview of Assays to Measure Specific Immunity
| TYPE OF IMMUNITY | ASSAYS |
|---|---|
| Humoral immunity | Antibody production: ELISA, cytometric bead array |
| Cellular immunity | Cytokines: ELISA, ELISPOT, cytometric bead array, RIA, intracellular cytokine staining |
| Cytotoxicity: 51 chromium release assay | |
| Proliferation: CFSE, lymphocyte proliferation assay | |
| Genetics | SNPs genetic microarray |
CFSE, carboxyfluorescein succinimidyl ester; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunosorbent spot; RIA, radioimmunoassay; SNP, single nucleotide polymorphisms.
BOX 19-1 Testing Methods
ELISPOT
Carboxyfluorescein succinimidyl ester
Carboxyfluorescein succinimidyl ester (CFSE) can be used to measure cell division. In this assay, the parent cell is incubated with CFSE, which is incorporated into DNA during cell division. As the cell divides, each of the daughter cells contains half of the CFSE stain. Every subsequent cell division halves the amount of CFSE in the daughter cells. When these cells are run through a flow cytometer, a characteristic pattern is seen (see Figure 19-2).
 Measurement of Antibodies
Measurement of Antibodies
Antibodies are essential to measuring immune function because they can act both as an indicator of disease and as a tool to measure the levels of other proteins and cells. Monoclonal antibodies are a powerful tool (see Box 19-2).
BOX 19-2 Monoclonal Antibodies
One of the primary tools used in many of the assays for immune function is the monoclonal antibody. Monoclonal antibodies are the product of a single B cell. To generate a monoclonal antibody, an antigen is injected into an animal, and the resultant B cells are isolated. These B cells are diluted, and a single B cell is fused with a nonsecreting myeloma cell line to form hybrids. These hybrids then produce the monoclonal antibodies highly specific for the injected antigen. In contrast, when any animal is injected with an antigen, polyclonal antibodies are generated. Many different B cells specific for that antigen release antibody, which is harvested and purified. This collection of antibodies is called polyclonal because, unlike monoclonal antibodies, it has many specificities to the same antigen. Polyclonal and monoclonal antibodies can be tagged with enzymes for fluorescence to detect the original protein antigens for which they are specific.1
Measuring serum antibody is essential in patients who have repeated or severe infections. Low antibody levels in these patients may indicate that they have an immunodeficiency. Patients with myelomas and lymphoproliferative disorders may have high antibody levels. For example, patients with alcoholic liver disease often have a polyclonal expansion of immunoglobulin-A (IgA), whereas patients with systemic lupus erythematosus (SLE) and Sjögren’s syndrome have polyclonal IgG expansion.1
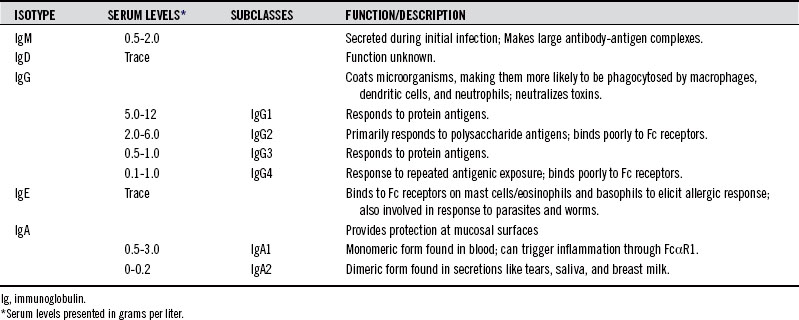
The type of isotype made by B cells can provide clues as to immunologic status of the patient (see Table 19-2).
IgG is the major antibody found in blood, and it consists of four subclasses, originally described in the 1980s according to their abundance in serum. IgG1 and IgG2 are present in much higher concentrations than IgG3 and IgG4. All subclasses of IgG are low in pediatric populations. Deficiency in some IgG subclasses results in increased susceptibility to bacterial infections.2 IgG subtypes vary in their ability to activate complement and Fc receptor binding. IgG3 has a strong affinity for Fc receptors and has the greatest ability to activate complement. The type of antigen can influence the subclass of antibody response. For example, IgG2 antibodies appear to influence polysaccharide antigens, whereas protein antigens and whole bacteria preferentially elicit IgG1.3–5
IgG4 antibodies, found at lowest concentration in the serum, are generated to repeatedly presented antigens. IgG4 deficient individuals may be more susceptible to pyogenic infections of the respiratory tract. Antibodies against dietary antigens are frequently IgG4. In a community survey of 40 individuals, anti-food antibodies to milk, egg, and fish of the IgG4 subclass were found in a significant proportion of a healthy population, indicating that IgG4 antibodies against food antigens cannot serve as markers of atopic disease. Because IgG4 cannot activate complement, it has been hypothesized that it may serve for protective clearance mechanisms and may be a desirable response to dietary antigens.6 IgG1 and IgG2 have also been found for dietary antigens, but the immunologic outcome of these subclasses may be food intolerance that is not caused by IgE mediated hypersensitivity.7 A larger discussion of the immune response to food can be found in Chapter 15.
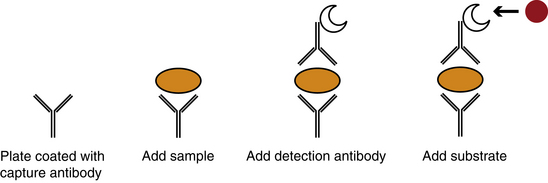
Antibodies of each of the IgG subclasses can be detected by a variety of techniques, including radioactive iodine labeling, antigen coated red cells, immunofluorescence with antigen coated sepharose, radioimmunoassay (RIA), and enzyme-linked immunosorbent assay (ELISA) with monoclonal antibodies (Figure 19-1).8
IgA can also be made to infectious agents and occurs in two forms, IgA1 and IgA2. IgA in serum predominantly exists in the monomeric form, IgA1. The ratio of IgA1:IgA2 in serum is about 9:1. IgA found in secretions, termed secretory IgA, occurs as dimers. The ratio of IgA1:IgA2 in secretions varies, but is approximately 6:4 in saliva.9 Because IgA is found at mucosal surfaces and lines the mucosal surface of the gut, it is not uncommon to find IgA antibody specific for food antigens. Because IgA is at high levels in secretions, it can be found in saliva in addition to serum, and salivary IgA tests are readily available.10,11
An immune response to parasites, specifically worms, triggers an IgE response.12 IgE elicits an immune response by binding to Fc receptors on mast cells, eosinophils, and basophils, causing degranulation and cytokine release. In atopic individuals, IgE is also made to allergens. IgE is at low levels in the blood. Measurement of total serum IgE is useful in patients in whom parasitic infection is suspected, but is not valuable for measuring allergies. Thus, the most common method for allergy testing is the skin prick test.13 In this test, a small amount of the suspected allergen is placed on the skin. Then the skin is pricked so that the allergen goes under the skin’s surface. If the patient is allergic to the allergen, swelling and redness will appear within 15 to 20 minutes.
Specific antibodies, rather than total levels, are also measured for circulating autoantibodies. These antibodies can be detected with immunofluorescence, RIA, and ELISA.14 Immunofluorescence involves examining the tissue directly with microscopy and is the least sensitive of these tests. The patient’s tissue is frozen and sections cut on a cryostat. This tissue is incubated with patient serum, such that autoantibodies in the serum can bind. A fluorescently tagged secondary antibody specific for human immunoglobulin is then used to detect the autoantibodies. Immunofluorescence examination of biopsy specimens of damaged or normal tissue may reveal deposits of immunoglobulins caused by antibodies reacting with an organ or tissue specific antigens. This approach is especially important in the diagnosis of antiglomerular basement antibody disease and bullous skin disorders, but may show false positives when used for SLE. (See Table 19-3 for a listing of common autoantibodies.)
TABLE 19-3 Common Autoantibodies
| AUTOANTIBODY | CLINICAL RELEVANCE |
|---|---|
| Antinuclear antibody | Systemic rheumatic diseases |
| Smooth-muscle antibody | Nonspecific liver damage; chronic hepatitis |
| Antimitochondrial antibody | Primary biliary cirrhosis |
| Endomysial antibody | Celiac disease; dermatitis; psoriasis |
| Antineutrophil cytoplasmic antibody | Vasculitis |
| Gastric parietal cell antibody | Pernicious anemia |
| Adrenal antibody | Idiopathic Addison’s disease |
| Pancreatic islet cell antibody | Insulin-dependent diabetes mellitus |
| Skin antibodies | Pemphigus vulgaris; Bullous pemphigoid |
| Antiacetylcholine receptor | Myasthenia gravis |
| Antimyelin basic protein | Multiple sclerosis |
| Double-stranded DNA autoantibody | Systemic lupus erythmatosus |
| Thyroid stimulating hormone antibody | Graves’ disease |
RIA is a far more sensitive test used for measuring antibodies that are in low concentrations. In RIA, an antibody specific for human antibody is tagged with a radioactive isotope. When this antibody is incubated with an autoantibody, the contact detects even low levels of autoantibody. ELISA is very similar to RIA; however, the enzymatic substrate is linked to the detection antibody.
Urine
When an antibody is made, the antibody light chains, κ and λ, are made in excess. These are present as free forms in serum and urine. Although small amounts of light chains are found in everyone, people with renal damage excrete higher levels of light chains in their urine. Free light chains are associated with malignant plasma dyscrasia and other lymphocyte-related immunoproliferative disorders. Intact immunoglobulin can also be found in urine.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree