Idiopathic and Diabetic Stiff Shoulder: Decision-Making and Treatment
Frances Cuomo
Evan L. Flatow
Jason A. Schneider
Julie Y. Bishop
F. Cuomo: Beth Israel Medical Center, Insall Scott Kelly Institute for Orthopaedics, New York, New York.
E. L. Flatow: Department of Orthopaedic Surgery, Mount Sinai Medical Center, New York, New York.
J. A. Schneider: Beth Israel Medical Center, Insall Scott Kelly Institute for Orthopaedics, New York, New York.
J. Y. Bishop: Department of Orthopaedic Surgery, Mount Sinai Medical Center, New York, New York.
INTRODUCTION
Frozen shoulder is one of the most common, yet poorly understood disorders of the glenohumeral joint. This is primarily due to the inability of physicians to agree on the exact definition of frozen shoulder syndrome, difficulty defining and differentiating it clearly from other conditions with similar symptoms and findings but with distinctly different etiologies, and confusing terminology. Frozen shoulder syndrome comprises a group of conditions with different underlying etiologies. Initially, periarthritis of the shoulder was used as an all-encompassing term to describe painful shoulders for which the symptoms could not be explained on the basis of arthritis of the glenohumeral joint.36 Codman described the disorder known as frozen shoulder as a “condition difficult to define, difficult to treat, and difficult to explain from the point of view of pathology.”28 The terms adhesive capsulitis and periarthritis of the shoulder are used, at times, with a meaning synonymous with frozen shoulder. In this chapter, a working definition of frozen shoulder is suggested: frozen shoulder is a condition of uncertain etiology characterized by significant restriction of both active and passive shoulder motion that occurs in the absence of a known intrinsic shoulder disorder.153
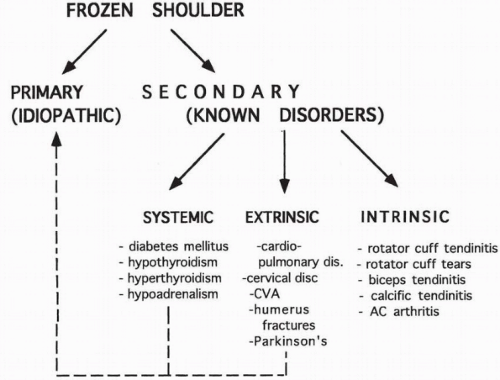
Previous authors have defined primary frozen shoulder as an idiopathic condition and secondary frozen shoulder as one associated with a known intrinsic, extrinsic, or systemic pathology.75 Although this is most likely a worthwhile distinction, it is probably inappropriate to include all intrinsic, extrinsic, and systemic etiologies as secondary frozen shoulder. Intrinsic disorders, such as rotator cuff pathology, should be considered separately because they represent a known underlying disorder or condition that results in the clinical picture of frozen shoulder. Extrinsic disorders, such as cervical radiculopathy or intrathoracic conditions, and systemic disorders, such as diabetes mellitus or hypothyroidism, should be considered together yet separate from the intrinsic disorders because they more closely resemble the primary or idiopathic condition. This schema is shown in Figure 10-1.
In this chapter, primary frozen shoulder, as well as secondary frozen shoulder due to the systemic cause of diabetes mellitus, will be discussed by reviewing pathophysiology, evaluation, operative and nonoperative management, and complications. Secondary frozen shoulder associated with intrinsic conditions and secondary etiologies, classified as extrinsic or other systemic causes, will not be discussed because, in these situations, treatment
approaches are directed primarily at correction of the underlying disorder, which is critical to the alleviation of the symptoms of frozen shoulder. Exclusion criteria for primary frozen shoulder, therefore, include patients with glenohumeral arthritis, fractures, dislocations, cervical spondylosis, neuromuscular disease, and referred pain from an intrathoracic source. Intrinsic shoulder pathology, such as subacromial impingement, calcific tendonitis, and postsurgical stiffness, therefore, is also excluded from this review and will be discussed elsewhere in the book.
approaches are directed primarily at correction of the underlying disorder, which is critical to the alleviation of the symptoms of frozen shoulder. Exclusion criteria for primary frozen shoulder, therefore, include patients with glenohumeral arthritis, fractures, dislocations, cervical spondylosis, neuromuscular disease, and referred pain from an intrathoracic source. Intrinsic shoulder pathology, such as subacromial impingement, calcific tendonitis, and postsurgical stiffness, therefore, is also excluded from this review and will be discussed elsewhere in the book.
SURGICAL ANATOMY AND BIOMECHANICS
When discussing frozen shoulder syndrome, it is important to include the overall shoulder girdle complex. The shoulder girdle complex consists not only of the glenohumeral joint but also of the acromioclavicular, sternoclavicular, and scapulothoracic articulations. Scapulothoracic and glenohumeral movements occur simultaneously as the arm is used away from the side.56 The normal glenohumeral to scapulothoracic ratio is 2:1, with the majority of elevation attributed to the glenohumeral joint.
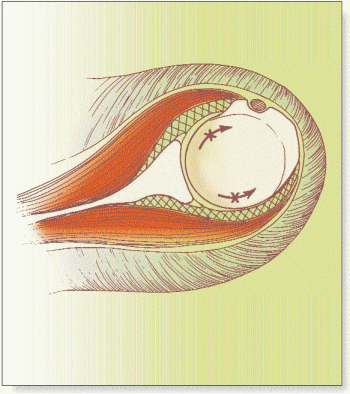
The inherently loose articulation of the normal shoulder is a necessary anatomic feature that permits the large range of motion required for normal shoulder function. The glenohumeral joint is enclosed entirely by the joint capsule. The capsule of the shoulder is normally a loose structure whose surface area is almost twice that of the humeral head.61 The stability of the shoulder is maintained further by osseous, static, and dynamic stabilizers. It is a nonconstrained articulation with the larger humeral head having nearly perfect congruity with the smaller osseous glenoid. To enhance the surface area of this loose articulation, the fibrous glenoid labrum is attached around the periphery of the glenoid. The dynamic stabilizers of the joint consist of the rotator cuff musculature and the long head of the biceps tendon. The rotator cuff muscles’ primary function is to create compression of the convex humeral head into the matched concavity of the glenoid fossa. The capsuloligamentous structures are the primary static stabilizers and are responsible for the stability of the joint, mainly at the extreme positions of rotation and translation because they are normally lax during most shoulder rotations. The capsule attaches around the perimeter of the glenoid and extends across to attach to the anatomic neck of the humerus, except inferiorly, where the attachment is about 1 cm distal to the articular margin. There are numerous areas within the capsule that are thickened secondary to attachment or confluence with local structures. The tendons of the rotator cuff adjacent to the joint capsule thicken the capsule anteriorly, superiorly, and posteriorly. In addition, the superior, middle, and inferior glenohumeral ligaments will present further areas of thickening of the joint capsule.11,12,14,34,40 The inferior capsule, however, is not supported by adjacent muscles and tendons, thereby leaving a lax double-fold of capsule, which forms the inferior or axillary recess. This potential axillary pouch space, formed by the redundant capsule fold, is normally present while the arm is at the side, but the pouch disappears and the capsule becomes taut as the arm is brought up to the forward elevation or abduction
allowed by the stretch of the inferior capsule. Superiorly, there is a triangular-shaped region between the anterior border of the supraspinatus tendon and the superior border of the subscapularis tendon known as the rotator interval. The ligamentous components of this region are the superior glenohumeral ligament and the coracohumeral ligament. Classic descriptions of the coracohumeral ligament describe it as a dense fibrous structure originating on the lateral surface of the coracoid process at its base and inserting into the greater and lesser tuberosities adjacent to the bicipital groove.34
allowed by the stretch of the inferior capsule. Superiorly, there is a triangular-shaped region between the anterior border of the supraspinatus tendon and the superior border of the subscapularis tendon known as the rotator interval. The ligamentous components of this region are the superior glenohumeral ligament and the coracohumeral ligament. Classic descriptions of the coracohumeral ligament describe it as a dense fibrous structure originating on the lateral surface of the coracoid process at its base and inserting into the greater and lesser tuberosities adjacent to the bicipital groove.34
At arthroscopy or arthrography, several normal recesses may be identified in the normal shoulder. Anteriorly, a synovial recess called the rotator cleft is often present between the superior and middle glenohumeral ligaments, also known as the subscapular bursa. There is also a posterior outpouching of the capsule, deep to the infraspinatus muscle, known as the infraspinatus bursa. There is an inferior recess of redundant capsule forming a pouchlike fold when the arm is at the side. The synovial membrane is also a structure implicated in the frozen shoulder process. This structure lines the capsule and is in continuity with the surrounding bursae and recesses. The synovium invests the long head of the biceps and passes deep to the transverse humeral ligament in the bicipital groove. The biceps tendon sheath may extend for a distance of 5 cm beyond the transverse ligament into the bicipital groove. The tendon sheath extends distally, especially when the arm is in abduction. This arm position is where the least amount of biceps tendon is intraarticular.85,86
During normal motion of the shoulder, the tightening and loosening of the glenohumeral ligaments and capsule encircling the humeral head is accompanied by lengthening and shortening of the rotator cuff and deltoid muscle.142 These structures, working in concert, allow for the normal 2:1 glenohumeral to scapulothoracic ratio necessary for the elevation of the arm. In the pathologic state of frozen shoulder syndrome, Nicholson noted that the scapula usually moved excessively in upward rotation to compensate for the loss of glenohumeral motion. On clinical examination, he consistently found the inferior glide of the humerus to be the most restricted of the accessory movements of the shoulder.98 Numerous investigators have described the pathologic findings associated with the frozen shoulder in an attempt to offer explanation for the observed microscopic capsular changes.28,75,77,78,87,91,92,107,117,132 Much of our current understanding of the pathology of frozen shoulder is due to the work of J. S. Neviaser, who coined the term “adhesive capsulitis” to describe an avascular, tense capsule that was markedly adherent to the humeral head and associated with decreased joint volume and synovial fluid. The histologic changes were consistent with chronic inflammation, fibrosis, and perivascular infiltration in the subsynovial layer, with the synovial layer remaining uninvolved.92
Simmonds theorized that a chronic inflammatory reaction caused by a degeneration of the supraspinatus tendon resulted in the capsular changes seen in frozen shoulder. He described a local hyperemia within the joint capsule, as observed earlier by Neviaser.132 He thought that the tight, fibrotic characteristics of the capsular tissue extended out into the soft tissues around the shoulder.
From intraoperative findings of gross contracture of the rotator cuff, McNab postulated that the primary lesion was in the cuff itself, with subsequent contracture of the capsule and coracohumeral ligament. He confirmed a region of constant hypovascularity in the supraspinatus tendon, called the critical zone by Codman,28 and theorized that this was responsible for the initial cuff degeneration.117 The localized degeneration of collagen was then theorized to induce an autoimmune response. McNab felt that the round cell infiltrate found in the capsule at open surgical release of the shoulder could be interpreted as an autoimmune response.117 Further reports have failed to identify any statistically significant clinical or laboratory evidence for an immunologic basis for this condition.75,85 Lundberg confirmed the absence of intra-articular adhesions in his operative observations at cinearthrography.74,75 Although at histologic examination synovial cells were found to be largely unchanged, Lundberg did report finding a more compact, dense collagen layer within the shoulder capsule. The glycosaminoglycans distribution in the shoulder capsule had the characteristics of a repair action.73
Ozaki and associates described the role of contracture of coracohumeral ligament and the rotator interval in the pathogenesis of frozen shoulder. These investigators found fibrosis, hyalinization, and fibrinoid degeneration in these structures.117 Neer also reported the importance of coracohumeral ligament contracture, but he stressed that it was unlikely that any one anatomic structure or pathologic process was responsible for causing the entire symptom complex associated with frozen shoulder.89, 90 and 91 The normal rotator interval contains elastic membranous tissue that, as a result, enhances the range of motion of the glenohumeral joint. The coracohumeral ligament helps to strengthen this region and acts as a suspensory ligament of the humeral head. The long head of the biceps is located beneath the rotator interval. In patients with chronic severe adhesive capsulitis, the pathologic findings in the coracohumeral ligament and in the rotator interval are extremely important. These structures are contracted and can be converted into a thick fibrous cord that holds the humeral head tightly against the glenoid fossa and restricts glenohumeral movement in all directions.
Much of the information regarding the gross and microscopic pathologic findings has been derived from operative observation and autopsy studies. DePalma described the fibrous capsule, synovial tissue, and rotator cuff as thickened, fibrotic, and contracted around the humeral head. The folds in the capsule and the synovial membrane along the inferior aspect of the humeral head were obliterated.33
Recent arthroscopic evaluation of patients with arthrographically documented adhesive capsulitis is believed by some authors to involve four stages of the disease. Stage 1, the preadhesive stage, is seen in patients with minimal or no motion limitation. There is a fibrinous synovial inflammatory reaction that is only detectable by arthroscopy. This reinforces the need to obtain arthroscopic evaluation of any patient who is treated by decompression for an impingement syndrome. In stage 2, acute adhesive synovitis, there is proliferative synovitis and early adhesion formation. The adhesions are seen well in the dependent folds extending to the humeral head. Stage 3, the stage of maturation, has less synovitis with loss of the axillary fold. Stage 4, the chronic stage, has adhesions that are fully mature and markedly restrictive.97
PATHOPHYSIOLOGY
The syndrome of frozen shoulder was first described by Duplay in 1896. He introduced the term “scapulo-humeral periarthritis” and felt that the initiating lesion was an obliteration of the subdeltoid bursa.36 Since then, numerous pathologic mechanisms have been proposed to explain the cause of frozen shoulder syndrome, all of which still remain largely theoretical. Myer suggested that the initiating lesion was a breakdown on the intra-articular portion of the biceps tendon.88 His observations were supported subsequently by Pasteur111 and Lippman68 and, more recently, by De-Palma.32 Codman, however, felt that the changes in the biceps tendon were of little etiological significance.28
McLaughlin, one of the earliest investigators to describe the changes in the rotator cuff, stressed the importance of contracture of the subscapularis in the development of the syndrome.81,82 Bateman reported his observations on the development of a hypertrophic inflammatory synovitis associated with intra-articular adhesions.5 Several investigators have proposed an autoimmune basis for frozen shoulder.7,21,22,23,50 Although some clinicians have reported a high incidence of HLA B27 in patients with frozen shoulder,22 others have not confirmed this association.61,100,124,137 In later studies, serum immunoglobulin A (IGA) levels were found to be significantly lower in patients with frozen shoulder, while the immune complex and C-reactive protein levels were increased.7,21,23 In general, however, sufficient evidence to support an immunologic therapy has been lacking.
A relationship to myofascial pain syndrome has been proposed. A syndrome of active trigger points about the shoulder, specifically within the subscapularis muscle, has been suggested as a possible cause of frozen shoulder syndrome.138 Trigger points are defined as locally tender, self-sustaining, hyperirritable foci located in the skeletal muscle or its associated fascia. The trigger points are also characteristically related to a zone of referred pain when the trigger is stimulated. Once activated, perpetuating factors may be responsible for the chronicity of pain. Another characteristic of the myofascial pain syndrome is palpable bands of muscle fibers that undergo a local twitch response when the trigger point is stimulated with a snapping palpation.14,133,149
Travell and others theorized that the subscapularis trigger points exert an influence on the sympathetic vasomotor activity, leading to hypoxia of the periarticular tissues. It is further theorized that the hypoxia leads to a local proliferation of fibrous tissue about the shoulder capsule, resulting in the clinical picture of frozen shoulder syndrome.88,138 A biochemical basis for frozen shoulder has been proposed. Lundberg, in his analysis of the capsule on patients with frozen shoulder, found an increase in glycosaminoglycans and a decrease in glycoprotein content. These biochemical changes in the capsule, however, are consistent with the process of fibrosis, and they may represent the effect of frozen shoulder rather than its cause.73,75
Neurologic dysfunction has been postulated as a cause of frozen shoulder syndrome. In 1959, Kopell proposed suprascapular compression neuropathy as a possible cause of frozen shoulder, but electromyography and nerve conduction studies have not supported this theory.63 Others have suggested that frozen shoulder is a result of autonomic dysfunction and represents a form of reflex sympathetic dystrophy.123 Sufficient evidence to support these hypothesis has not been provided.
Bunker et al. prospectively studied 50 patients with the diagnosis of primary frozen shoulder. These authors were able to identify increased serum lipid levels in these patients compared to age- and sex-matched control subjects. The fasting serum triglyceride and cholesterol levels were significantly elevated in the frozen shoulder group. Increased serum triglyceride levels have also been found in patients with diabetes and with Dupuytren’s disease, suggesting that hyperlipidemia may be the common thread that links these three disorders.24,25
Various endocrine disorders are associated with frozen shoulder. In particular, patients with diabetes manifest a much greater incidence of frozen shoulder than their nondiabetic counterparts.17 Frozen shoulder has also been reported to occur with increased incidence among patients with thyroid disorders, hypoadrenalism, and corticotrophin deficiency.13,39,148,26
Trivial trauma has been postulated to be an important factor, particularly when it is followed by a prolonged period of immobilization.32,115 This does seem to be the sequence of events in some patients who develop frozen shoulder. The association of frozen shoulder with major trauma to the shoulder or other parts of the upper extremity is recognized. The association with minor trauma, which may be forgotten, is difficult to document and may be overlooked.28,82 Most patients who sustain minimal trauma, even when combined with a period of immobilization,
do not develop frozen shoulder. This has led some investigators to conclude that there are some patients who possess a “constitutional” predisposition for the development of frozen shoulder. Support for this theory is provided by the significant incidence of bilateral frozen shoulders.22,75,101,119,151
do not develop frozen shoulder. This has led some investigators to conclude that there are some patients who possess a “constitutional” predisposition for the development of frozen shoulder. Support for this theory is provided by the significant incidence of bilateral frozen shoulders.22,75,101,119,151
The role of psychologic factors has been considered in the development of frozen shoulder. Some investigators have suggested that a certain personality structure, coupled with untoward life events and inappropriate response to stress, may serve as a predisposing or precipitating factor for the development of frozen shoulder.29,39,71,123 Coventry chose the term periarthritic personality to describe one component of a three-part theory on the pathogenesis of frozen shoulder in a group of patients with painful stiff shoulders. He observed that most patients had “a peculiar emotional constitution in which they were unable to tolerate pain, expected others to make them well, and refused to take personal initiative in their recovery.”29 Other studies, however, have found no evidence for a characteristic personality disorder.101,151 It would, therefore, appear that a specific periarthritic personality type is difficult to identify. The role of psychologic factors should be considered, at best, a secondary factor in the management of these patients.
Fibromatosis has also been implicated in causing frozen shoulder syndrome. The pathomechanics are believed to be found in fibrous tissue contracture formed in response to cytokines, lymphocytes, or monocyte products. Plateletderived growth factor is a potent mytogenic polypeptide for mesenchymal cells. Immunocytochemistry was performed with monoclonal antibodies on the rotator ligament excised from 12 patients with resistant frozen shoulder. Bunker and Anthony report that the pathologic process is active fibroblastic proliferation accompanied by some transformation to a smooth muscle phenotype (myofibroblasts). The fibroblasts lay down collagen, which appears as a thick nodular band or fleshy mass. These appearances are reportedly very similar to those seen in Dupuytren’s disease of the hand, with no inflammation and no synovial involvement. The contracture acts as a checkrein against external rotation, causing a loss of both active and passive movement.24
Frozen shoulder associated with a known underlying disorder is considered to be secondary, and this group includes intrinsic, extrinsic, or secondary disorders. Intrinsic shoulder abnormalities include rotator cuff tears, tendonitis of the long head of the biceps tendon, calcific tendonitis, and acromioclavicular arthritis. Extrinsic disorders, which represent pathologic conditions apart from the shoulder region, include ischemic heart disease and myocardial infarction83,151; pulmonary disorders including tuberculosis,59 chronic bronchitis, emphysema,130 and tumor31; cervical disc disease and radiculopathy4,60,151; cerebral vascular hemorrhage16,19; previous coronary artery bypass graft surgery133; previous breast surgery; lesions of the middle humerus134; and central nervous system disorders, such as Parkinson’s disease.122 Systemic disorders represent generalized medical conditions that are known to occur in association with frozen shoulder. Such conditions and poor prognostic indicators include diabetes mellitus, hypothyroidism, hyperthyroidism, and hypoadrenalism.
EPIDEMIOLOGY AND NATURAL HISTORY
Epidemiologically, the exact prevalence and incidence of frozen shoulder are not known; however, the cumulative risk of at least one episode of frozen shoulder has been estimated to be a minimum of 2%.75 It is most frequently found in patients between the fourth and sixth decades of life, and it is more common in women than men.4 The nondominant extremity appears to be more commonly involved, with most reported cases described as affecting the left side.32,68,75 Bilateral involvement occurs in 6% to 50% of cases, although only 14% of these bilateral cases manifest simultaneously.4,7,23,75,123 When a history of bilateral involvement is identified, the possibility of a constitutional predisposition should be explored.5,75,123 With adhesive capsulitis, the same shoulder is rarely involved again.7,8
There is significant controversy over the natural history of frozen shoulder with respect to both objective and subjective outcomes. Historically, frozen shoulder has been touted as a condition in which “recovery is always sure and maybe confidently expected.”28 Several investigators using a variety of treatment methods have reported a high percentage of affected patients achieving full range of motion.28,42,68 In addition, they have found complete or near complete symptomatic relief.42,50 More recent investigations have questioned the early optimistic reports, finding measurable restriction at follow-up in 39% to 76% of patients20,27,83,87,119 and persistent symptoms in up to 45%.7,115
The time course of adhesive capsulitis has been described as classically lasting 18 to 24 months.7 Recent studies have challenged this commonly held belief. Reeves noted that the mean duration of symptoms was 30 months.119 Patients describing themselves as functionally recovered tend to underestimate their loss of motion.20 Reeves described some shoulder motion restriction in over 50% of patients in a 5- to 10-year follow-up, but functional impairment was identified in only 7%.119 Clark found that 42% of patients had persisting limitations of motion after 6 years of follow-up.27 Binder, in a prospective study, noted that 90% of patients did not regain the minimum range of motion when matched for age and sex with a controlled group 6 months after diagnosis. He also reported that 40% of patients failed to regain a minimum range of motion when matched for age and sex with a controlled group
when followed for a minimum of 3 years.7 In a retrospective study of a carefully selected group of frozen shoulder patients performed by Schaffer et al., almost half remained symptomatic many years after the onset of symptoms, and up to 56% had residual restriction in one or more planes.130
when followed for a minimum of 3 years.7 In a retrospective study of a carefully selected group of frozen shoulder patients performed by Schaffer et al., almost half remained symptomatic many years after the onset of symptoms, and up to 56% had residual restriction in one or more planes.130
Despite the subjective and objective outcome of this disorder, there seems to be widespread agreement with regard to the seeming lack of significant or frequent functional disability documented at cessation of treatment. Regardless of objective restriction or the presence of symptoms, few patients are reportedly functionally restricted to any significant degree.7,27,69,119 The lack of correlation between subjective and objective findings has been noted consistently.7,9,20,27,50,87 Symptomatic patients frequently have no measurable restricted range of motion in any plane. Conversely, those patients with the most significant motion were often pain-free. However, whether this difference is from the patient adapting to the restricted motion or due to the restricted motion not being present in daily living activities is an unresolved issue. According to Neer, however, the presence of such restriction depends upon the functional demands of the patient.91 Even in the active patient, the presence of 150 degrees of active elevation, 50 degrees of external rotation, and internal rotation to the eighth thoracic vertebras is probably sufficient for normal function. In Schaffer’s report of an older population whose functional demands were surely less than the demands just stated, the degree of restriction tolerated in any plane was certainly even greater. The authors state that the preeminent importance of forward flexion and elevation in daily activities superseded the findings of restriction predominantly in the abducted and externally rotated positions, which resulted in little functional impairment.130
In general, the natural history of frozen shoulder is uncertain, and additional randomized, prospective studies are needed. The difficulty of performing these studies, however, is because of the ethical dilemma of assigning patients to an untreated group.
DIABETES MELLITUS
Patients with diabetes mellitus, in particular, manifest a much greater incidence of frozen shoulder than their nondiabetic counterparts. The incidence of frozen shoulder in diabetic patients is approximately 10% to 20%, but may be as high as 36%.17,67,75,151 Bridgman found that the incidence of frozen shoulder in 800 diabetic patients was 10.8%, compared with 2.3% in 600 nondiabetic controls.17 Diabetic patients have a significantly higher incidence of bilateral involvement, with bilateral presentation in as many as 77% of those cases.127 Generally, frozen shoulder is noted more frequently in patients with long-standing insulin-dependent diabetes mellitus (IDDM).2,103,108,125 It is generally believed that the long-term use of supplemental insulin and fluctuating levels of glucose increase the risk of developing shoulder stiffness.84 The high blood-glucose levels are thought to accelerate aging of certain body proteins, which trigger a series of chemical reactions in proteins. This eventually leads to cross-linking between adjacent molecules, such as long collagen chains.2,84,128 This makes the collagen more resistant to degeneration and more likely to accumulate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree