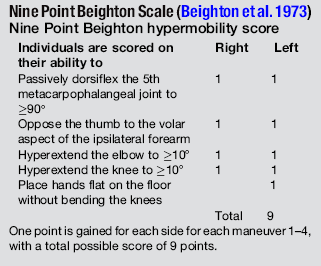
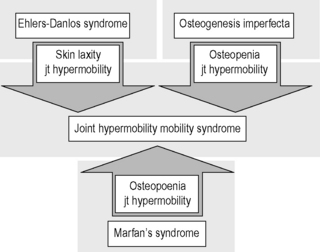
6.3 Hypermobility and the hypermobility syndrome The first clinical description of hypermobility has been attributed to Hippocrates in the 4th Century BCE, who described the Scythians, a central European tribe, as having such flabbiness and atony, that they lost battles due to their inability to draw their bows and arrows effectively because of their unstable shoulders and elbows (Beighton et al. 1999). While hypermobility did not feature again in medical writings until the 19th century, Matthias Grunewald (1460–1528) observed hypermobility in “Saint Cyriaque” in the Heller Retable (Fig. 6.3.1), and later, Peter Paul Rubens observed hyperextension of the metacarpal joints, flat-footedness and hyperlordosis in “The Three Graces” (1638–1640; Prado, Madrid). Furthermore, the musical successes of Paganini were attributed to his extreme hand mobility, in the 18th century (Larsson et al. 1993). Fig. 6.3.1 • Matthias Grunewald (1460–1528) observed hypermobility in “Saint Cyriaque”. From Matthias Grünewald in Wikipedia. Available from http://en.wikipedia.org/wiki/Matthias_Gr%C3%BCnewald (accessed 16 August 2011). Joint hypermobility is a unifying feature of the heritable disorders of connective tissue (HDCT), (Beighton et al. 1999) a group of genetic disorders affecting connective tissue matrix proteins identified in the 19th century. These include the Ehlers–Danlos syndrome (EDS), Marfan syndrome (MFS) and osteogenesis imperfecta (OI). In recent years a more common, less serious connective tissue disorder with a mixed phenotype has been proposed, termed the joint hypermobility syndrome (JHS) and considered to be an atypical or forme fruste of HDCT, and sharing overlapping features with EDS, MFS and OI (Fig. 6.3.2). Some authorities consider JHS to be synonymous with EDS type III (Grahame 2003). Fig. 6.3.2 • Interrelationship between the hereditary connective tissue disorders. Adapted from Grahame, 2003. The HDCTs share many common features of chronic musculoskeletal pain, soft tissue and visceral injury, cardiovascular pathology and dysfunction, skin abnormalities, fatigue and neurogenic dysfunction (Bird 2007; Grahame 2009). Genetic and histologic investigations do not differentiate the conditions and therefore the diagnosis is made on clinical grounds, such as echocardiography to delineate cardiovascular involvement, and slit-lamp examination to establish ocular involvement (Hakim & Grahame 2003a). Although the cardinal features of EDS, NFS and IO include hyperextensible skin, marfanoid body shape, and brittle bones, respectively, these features are not pathognomic to each condition. Hakim & Grahame (2003a) stress that hypermobility of joints is also common to all, with subtle differences and similarities between the HDCTs. JHS is a genetically inherited disorder, presenting with an autosomal dominant pattern, thought to affect the encoding of the connective tissue protein collagen (Grahame 2003). Individuals with JHS display an abnormal ratio of type III to type I collagen (Child 1986). Type I collagen has a high tensile strength and is the most common collagen in the body, abundant in tendon, joint capsule, skin, demineralized bone, and nerve receptors. Type II collagen is found in cartilage, and designed to withstand compressive stress, whereas type III collagen is much more extensible and disorganized, occurring in organs such as the gut, skin, and blood vessels (Beighton et al. 1999), which may explain the inherent laxity or ‘reduced tissue stiffness’ (Russek 1999). Mutations in genes encoding collagen type V have also recently been implicated (Malfait et al. 2005), with type V collagen under normal control interacting with type I collagen during fibrillogenesis and having a role in regulation of fibril diameter. A mutation in tenascin-X, a noncollagenous molecule, has also been suggested as contributing to joint hypermobility. An alteration in this process may potentially lead to thinner, fine, and more disorganized collagen fibers. Skin fibroblast biopsy analysis has allowed researchers to further investigate the microscopic structural discrepancies that may define HCTDs. Malfait and co-workers (2005) hypothesize that interference with the processing of the N-propeptide of either α-chain (α1 or α2) of type I collagen is responsible for EDS-like symptoms of skin laxity, joint subluxation, and dislocation. The Beighton nine point scale scoring system (see Box 6.3.1, Beighton et al. 1973) is an adaptation of the method first described by Carter & Wilkinson (1964) and validated for adult populations by Bird et al. (1979). The Contempassis tool is a more sensitive tool for measuring generalized joint hypermobility, but the Beighton scale is usually favored by clinicians because it is easy and quick to apply (Bird 2007). Both the Carter–Wilkinson and Beighton scales were developed for epidemiologic purposes and although widely used as a clinical assessment test for hypermobility are heavily biased towards the upper limb and pauciarticular hypermobility. Furthermore, a hypermobile joint falling outside of the five sampled joints may be missed (Hakim & Grahame 2003b). Adults are considered to be hypermobile if they have four or more hypermobile joints, while five or more joints is the suggested cut-off point in children (Rikken-Bultmann et al. 1997). The revised Brighton Criteria (see Box 6.3.2) were developed to clarify the relationship between hypermobility and musculoskeletal disorders (Grahame et al. 2000). The criteria were developed for research purposes but may also be used as a clinical tool to diagnose JHS. It is important to appreciate that a single symptomatic hypermobile joint is sufficient to satisfy the diagnosis of JHS. The 1998 Brighton Criteria take into account localized and/or pauciarticular hypermobility and this phenomenon is incorporated as a minor criterion for the diagnosis, along with a number of other connective tissue signs and symptoms. Hakim & Grahame (2003a) have recently developed a five part questionnaire (Box 6.3.3) for identifying hypermobility. This easy-to-use tool has demonstrated very good specificity and sensitivity, of 80% and 90%, respectively (Hakim & Grahame 2003b). The strength of this tool is that potentially it can be used to screen individuals with diffuse musculoskeletal symptoms in whom no clear cut degenerative or inflammatory disease has been identified (Hakim & Grahame 2003b). It also has excellent potential as a diagnostic tool in population epidemiologic studies, where physical examination is not possible or is impractical (Hakim & Grahame 2003a,b). Professor Bird stresses that while all these scoring systems are useful for screening purposes, they are not a substitute for a careful clinical examination of all of an individual’s joints and tissues (Bird 2007). The reported prevalence and incidence of hypermobility and JHS varies in the literature. It is difficult to compare studies because of variation in the screening and diagnostic criteria. Gender, ethnicity, and age are important factors, with hypermobility, being more prevalent in females and those of African or Asian descent (Bird 2007). While the populations with the most flexible joints appear to have fewer problems (Bird 2007), further population studies are required to establish this observation. Joint flexibility and hypermobility decrease with age (Grahame 2009). The prevalence of hypermobility in children is between 10 and 25%, with a higher incidence in females than males (Larsson et al. 1987). The prevalence of hypermobility in adults also varies, from as little as 5% in the USA (Jessee et al. 1980) to between 25% and 38% in Iraq and 43% being recorded in the Noruba tribe in Nigeria. Recent studies in clinical populations showed the JHS phenotype was present in 58% of females and 29% of males among nonCaucasians in a west London rheumatology clinic. Similarly, in multicultural Oman, 55% of female patients between 18 and 50 years attending the rehabilitation outpatient department exhibited the JHS phenotype (Clark & Simmonds 2011). Hypermobility does not necessarily result in problems and may sometimes be considered an asset, but can be the cause of a variety of debilitating symptoms (Grahame 2003). The reason why certain individuals develop symptoms and others do not is not yet clear. The degree of joint and skin laxity and bruising has some association with injury risk and is likely to be related to a number of biopsychosocial influences (Murray 2006). Testing skin laxity is an important aspect of the clinical assessment (Fig. 6.3.3). Fig. 6.3.3 • Testing skin laxity. Symptoms frequently commence in early childhood, with the potential to continue into adult life. The predominant presenting complaint is pain, which is often widespread and longstanding, ranging from 15 days to 45 years. As early as birth, specific problems have been associated with hypermobility, including dislocation of the hip. Kirk et al. (1967) reported three quarters of hypermobile adolescents developing symptoms by the age of 15. Murray & Woo (2001) and Adib et al. (2005) recognize JHS as one of the most frequent causes of musculoskeletal symptoms in children and adolescents, particularly girls, aged between 13 and 19 years. Tofts et al. (2009) affirm this, stating: Virtually all parts of the musculoskeletal system may be affected in JHS, with particular problems occurring at different ages from a combination of developmental changes or differences in growth patterns and the degree of physical activity. Individuals frequently look well and move well, which does not match complaints (Russek 1999; Simmonds & Keer 2007). This may lead to the patient being misunderstood and made to feel like a hypochondriac or labeled as having psychological problems (Child 1986). In children, lower limb arthralgia is the commonest presentation (Murray 2006). These pains are usually biomechanical in origin and provoked by prolonged weightbearing and may be associated with periarticular joint swelling. Benign nocturnal pains, so-called “growing pains”, are common in childhood and have been linked to hypermobility. It has been suggested that these pains are a result of minor injury following unusual or excessive physical exercise and sport (Murray & Woo 2001). Persistent pain can lead to avoidance behavior which can precipitate a downward spiral of deconditioning, leading to reduced functional capacity and confidence. Laxity of ligaments predisposing to joint instability, subluxation, and dislocation is commonly reported (Hakim & Grahame 2003a). Congenital hip problems have been linked with hypermobility, most frequently recurrent subluxations (“clicky hips”), and less often hip dysplasia (Adib et al. 2005; Murray 2006) Such clicking of joints is also commonly reported in many other joints as children get older, both spontaneously and as habitual cracking of joints of the hand, temporomandibular joint, and spine. The origin of this is thought to be the formation and collapse of “air bubbles” in the joint, with sudden “excessive” distension of the joint spaces. Back pain is another common presentation in both children and adults which should be thoroughly investigated, particularly in children and adolescents (Murray 2006). Hypermobility syndrome is one of the most common differential diagnoses in this population (Grahame 1999). Spinal pain frequently presents in adolescents and may be related to growth spurts and associated biomechanical changes. The pain is often a result of muscle spasm and can be associated with scoliosis which may extend throughout the spine. It may also be related to excessive unaccustomed physical activity (Simmonds & Keer 2007). Conversely, obesity, excessive sedentariness, and poor fitness may also be contributory factors. These disorders may be the forerunner of chronic adult low back pain and cervical pain disorders. Where pain is severe or disabling, then other diagnoses need to be considered (i.e., spondylolysis, spondylolisthesis) and these are also more likely in hypermobile subjects. In young adults with hypermobility, disc prolapse as well as early degenerative osteoarthritis also need to be considered (Murray 2006). Joint hypermobility is a risk factor for premature osteoarthritis in the hands, knees and spine (Bird et al. 1978). Postulated mechanisms include joint instability and overuse, errors in collagen genes, or linkage between the genes for osteoarthritis and collagen abnormalities. Jónsson et al. (1996) observed associations with osteoarthritis of the hands, in particular the thumb.
Assessment and management
Introduction

Pathogenesis
Testing for hypermobility and hypermobility syndrome
Marfan’s syndrome
Clinical presentation of hypermobility syndrome
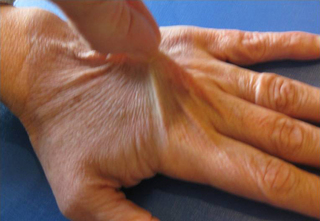
One suggested method is to draw the skin up from the 3rd metacarpal. The transparency of the skin can also be noted.
Musculoskeletal signs and symptoms
Osteoarthritis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Hypermobility and the hypermobility syndrome: Assessment and management