Hemiarthroplasty of the Distal Humerus
Rick F. Papandrea
Editor’s note: At the time of publication, the use of a hemireplacement is considered as an “off-label” identification by the FDA. The material is being presented as we find the concept has merit.
INTRODUCTION
Like many orthopaedic procedures, hemiarthroplasty is an old idea that has gained resurgence because of improved implants and a better understanding of the indications and related pathology. The reemergence of this technique had been possible due to the availability of anatomically shaped distal humeral components. These components are either the humeral component of an unlinked total elbow arthroplasty system (Sorbie-Questor, Wright Medical, Arlington, TN) or a custom component (spool) for a convertible elbow arthroplasty (Latitude, Tornier, Saint-Ismier, France). Currently, there is no dedicated hemiarthroplasty for the distal humerus, nor is there commercial support for using the available implants in this manner. Furthermore, unfortunately, at the time of this publication, neither of the above designs, the use of which is reported here, is currently available for this purpose in the United States.
In the United States, the FDA (Food and Drug Administration) approves implants for marketing for specific purposes. Both of the available implants have not been approved for use in hemiarthroplasty. This means that use of the implants for hemiarthroplasty is considered “off-label.” It is legal and ethical to utilize implants in an off-label manner, but the surgeon should disclose this information to the patient. The companies, on the other hand, cannot advertise or promote such use of these implants.
Hemiarthroplasty can be utilized for both acute and chronic conditions. If there is any way to reconstruct a joint with native tissue, this is preferred to prosthetic reconstruction. Prosthetic reconstruction of the elbow should be considered when all other nonimplant options have been exhausted or deemed inappropriate.
The role of hemiarthroplasty is developing. It can fill the void between reconstruction and total elbow replacement. The role of interposition prior to prosthetic arthroplasty is debatable and will not be covered.
INDICATIONS
The indications for hemiarthroplasty are still being defined.
The requisites for consideration are that the proximal radius and ulna should be normal, or nearly normal, both in cartilage coverage and anatomic shape.
The elbow must be stable, or the surgeon must be confident that stability can be gained by repair or reconstruction.
Acute indications for hemiarthroplasty include supracondylar, intercondylar, capitellar, and shear fractures that are not reconstructable. While an experienced elbow surgeon may be able to determine the ability to reconstruct a fracture based on preoperative images, it is imperative that the concept of hemiarthroplasty for fracture treatment may take the place of a skilled effort to reconstruct the native anatomy. The clinical experience with hemiarthroplasty is growing, but still too limited to state definitely that any one type of fracture of any specific clinical situation is acutely best treated by hemiarthroplasty over fracture fixation. Comminuted articular shear fractures may be the best indication recognized to date. It is most prudent to have a hemiarthroplasty available when considered but attempt to reduce and rigidly fix a fracture, converting to hemiarthroplasty only when the fracture is deemed unreconstructable.
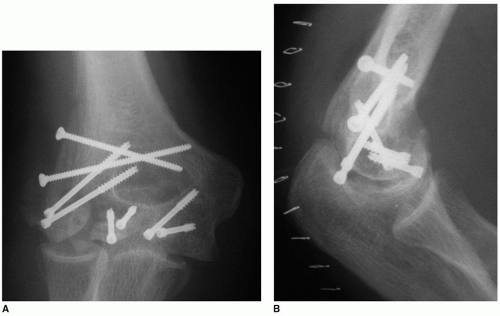
Subacute and chronic situations are clearer indications for hemiarthroplasty. Hemiarthroplasty should be considered after failed internal fixation when repeat fixation is determined to be impossible (Fig. 34-1). Distal nonunion of a low supracondylar fracture is another indication for a hemiarthroplasty. Since motion may occur through the nonunion site, there may be intra-articular adhesions that cause cartilage delamination during attempted reconstruction.
Although uncommon, avascular necrosis (AVN) presents another chronic indication for hemiarthroplasty. Given the low incidence of AVN in the elbow, there is no literature to guide the treatment. If lesser procedures fail, or the AVN has caused collapse of the subchondral bone with chondral fractures and damage, hemiarthroplasty is a reasonable salvage. This most often only involves the capitellum; hence an isolated capitellar replacement might be considered (see Chapter 33).
CONTRAINDICATIONS
Active infection is an absolute contraindication to hemiarthroplasty. History of infection should be considered to be a relative contraindication. The native proximal radial head and the olecranon must be relatively normal to allow for proper articulation without pain. Structural abnormalities to the proximal radius and ulna should be considered to be contraindication to hemiarthroplasty.
While a minimal amount of cartilage loss may be tolerated articulating against the metallic distal humeral component, severe cartilage loss will most likely limit the ability to have a pain-free articulation.
Previous radial head resection (or an unreconstructable radial head fracture) should be considered to be a relative if not absolute contraindication to ulnohumeral replacement. Expecting the articulation to work appropriately with just a proximal ulna, even if in pristine condition, may be too optimistic. This author is not aware of any cases utilizing a prosthetic radial head articulating with a native proximal ulna and a hemiarthroplasty.
PREOPERATIVE PLANNING
One should be certain that there are no concomitant injuries that would affect the outcome of a hemiarthroplasty. As noted above, any history of infections or medical history that may influence the risk of infection should be investigated.
Previous operative notes should be reviewed, any existing hardware needs to be considered, and appropriate equipment to remove existing hardware, if necessary, should be available.
The nerve function of the limb should be documented, and the soft tissue envelope needs to be examined to ensure that standard incisions are appropriate or alternative approaches can be carried out.
Proper imaging may be the most important aspect of the preoperative planning. Standard radiographs may be enough to determine that a hemiarthroplasty is appropriate and carry out the procedure. Oblique or traction views may add information.
At times, the determination of appropriate reconstruction for a distal humerus fracture or chronic condition may require a CAT scan to determine if the proximal radius and ulna are involved in such a way to preclude hemiarthroplasty, or if an injury cannot be reconstructed without nonprosthetic methods. We have found that 3D images offer a much more precise understanding of the pathology.
Note: As long as the axial data obtained from the CAT scan are available in DICOM format, the treating clinician may create 3D images using open source software (1).
SURGERY
Patient positioning can be either supine or lateral. Lateral positioning is carried out with an arm positioner that supports the brachium. If a supine position is chosen, and no arm table is utilized, an over-the-body support may minimize the need for additional assistance during the procedure.
Note: This author prefers to use the supine position, with a commercially available arm positioner, and no hand table. This allows unrestricted use of a small C-arm for imaging. A small “bump” of folded surgical bowels will lift the shoulder to aid in draping and prepping. Unless there is proximal hardware or fracture that requires posterior dissection of the radial nerve, a nonsterile tourniquet is placed as high on the brachium as possible.
A standard posterior skin incision to one side of the olecranon is typically utilized. Full-thickness skin flaps are elevated medially and laterally. The ulnar nerve needs to be identified and “controlled” prior to mobilizing the medial flap too far.
Note: Previously operated elbows may or may not have a transposed nerve. “Assume nothing.” A nerve that has been transposed may take a tortuous course coming back upon itself due to the additional length of nerve afforded by its nonanatomic position.
Once safely identified, the nerve should be dissected to allow a subcutaneous transposition (Fig. 34-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree